Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation
- PMID: 23162013
- PMCID: PMC3569043
- DOI: 10.1161/ATVBAHA.112.300399
Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation
Abstract
Objective: Genomewide association studies have implicated allelic variation at 9p21.3 in multiple forms of vascular disease, including atherosclerotic coronary heart disease and abdominal aortic aneurysm. As for other genes at 9p21.3, human expression quantitative trait locus studies have associated expression of the tumor suppressor gene CDKN2B with the risk haplotype, but its potential role in vascular pathobiology remains unclear.
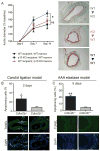
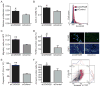
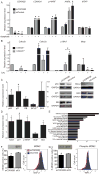
Methods and results: Here we used vascular injury models and found that Cdkn2b knockout mice displayed the expected increase in proliferation after injury, but developed reduced neointimal lesions and larger aortic aneurysms. In situ and in vitro studies suggested that these effects were attributable to increased smooth muscle cell apoptosis. Adoptive bone marrow transplant studies confirmed that the observed effects of Cdkn2b were mediated through intrinsic vascular cells and were not dependent on bone marrow-derived inflammatory cells. Mechanistic studies suggested that the observed increase in apoptosis was attributable to a reduction in MDM2 and an increase in p53 signaling, possibly due in part to compensation by other genes at the 9p21.3 locus. Dual inhibition of both Cdkn2b and p53 led to a reversal of the vascular phenotype in each model.
Conclusions: These results suggest that reduced CDKN2B expression and increased smooth muscle cell apoptosis may be one mechanism underlying the 9p21.3 association with aneurysmal disease.
Figures


Similar articles
-
CDKN2B Regulates TGFβ Signaling and Smooth Muscle Cell Investment of Hypoxic Neovessels.Circ Res. 2016 Jan 22;118(2):230-40. doi: 10.1161/CIRCRESAHA.115.307906. Epub 2015 Nov 23. Circ Res. 2016. PMID: 26596284 Free PMC article.
-
MFAP4 Promotes Vascular Smooth Muscle Migration, Proliferation and Accelerates Neointima Formation.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):122-33. doi: 10.1161/ATVBAHA.115.306672. Epub 2015 Nov 12. Arterioscler Thromb Vasc Biol. 2016. PMID: 26564819
-
H19 Induces Abdominal Aortic Aneurysm Development and Progression.Circulation. 2018 Oct 9;138(15):1551-1568. doi: 10.1161/CIRCULATIONAHA.117.032184. Circulation. 2018. PMID: 29669788 Free PMC article.
-
Genetics of abdominal aortic aneurysm.Curr Opin Cardiol. 2013 May;28(3):290-6. doi: 10.1097/HCO.0b013e32835f0d55. Curr Opin Cardiol. 2013. PMID: 23478885 Review.
-
The p53 pathway in vasculature revisited: A therapeutic target for pathological vascular remodeling?Pharmacol Res. 2021 Jul;169:105683. doi: 10.1016/j.phrs.2021.105683. Epub 2021 May 18. Pharmacol Res. 2021. PMID: 34019981 Review.
Cited by
-
Skeletal muscle transcriptome in healthy aging.Nat Commun. 2021 Apr 1;12(1):2014. doi: 10.1038/s41467-021-22168-2. Nat Commun. 2021. PMID: 33795677 Free PMC article.
-
Genetics and Genomics of Coronary Artery Disease.Curr Cardiol Rep. 2016 Oct;18(10):102. doi: 10.1007/s11886-016-0777-y. Curr Cardiol Rep. 2016. PMID: 27586139 Free PMC article. Review.
-
Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives.Curr Med Sci. 2024 Feb;44(1):1-27. doi: 10.1007/s11596-023-2818-2. Epub 2023 Dec 7. Curr Med Sci. 2024. PMID: 38057537 Review.
-
Potential Mechanisms and Effects of Efferocytosis in Atherosclerosis.Front Endocrinol (Lausanne). 2021 Feb 1;11:585285. doi: 10.3389/fendo.2020.585285. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33597922 Free PMC article. Review.
-
Walking impairment questionnaire improves mortality risk prediction models in a high-risk cohort independent of peripheral arterial disease status.Circ Cardiovasc Qual Outcomes. 2013 May 1;6(3):255-61. doi: 10.1161/CIRCOUTCOMES.111.000070. Epub 2013 Apr 30. Circ Cardiovasc Qual Outcomes. 2013. PMID: 23633217 Free PMC article.
References
-
- Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. JAMA. 2004;291:2204–2211. - PubMed
-
- Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. - PubMed
-
- Musunuru K, Kathiresan S. Genetics of coronary artery disease. Annu Rev Genomics Hum Genet. 2010;11:91–108. - PubMed
-
- Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL103605/HL/NHLBI NIH HHS/United States
- R01 HL38854/HL/NHLBI NIH HHS/United States
- K08 HL103605-01/HL/NHLBI NIH HHS/United States
- R01HL103635/HL/NHLBI NIH HHS/United States
- R01 HL087867/HL/NHLBI NIH HHS/United States
- T32 HL094274/HL/NHLBI NIH HHS/United States
- T32 HL098049/HL/NHLBI NIH HHS/United States
- R01 HL57353/HL/NHLBI NIH HHS/United States
- R01 HL038854/HL/NHLBI NIH HHS/United States
- K12 HL087746/HL/NHLBI NIH HHS/United States
- R01 HL057353/HL/NHLBI NIH HHS/United States
- R01 HL121008/HL/NHLBI NIH HHS/United States
- R01 HL103635/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

