Scaleable processes for the synthesis of (-)-β-D-2,6-diaminopurine dioxolane (Amdoxovir, DAPD) and (-)-β-D-2-aminopurine dioxolane (APD)
- PMID: 23162170
- PMCID: PMC3496293
- DOI: 10.1016/j.tet.2012.05.039
Scaleable processes for the synthesis of (-)-β-D-2,6-diaminopurine dioxolane (Amdoxovir, DAPD) and (-)-β-D-2-aminopurine dioxolane (APD)
Abstract
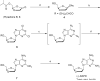
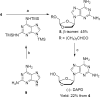
An efficient and scalable synthesis of (-)-DAPD and (-)-APD has been developed. We discovered that t-butyl cyanoacetate can be used as a new additive for the sugar nucleoside base coupling step en route to DAPD with improved β-selectivity and an isolated yield four fold greater than the original process scale method. Using this new process, (-)-DAPD has been prepared on greater than 20 g scale. In the synthesis of (-)-APD, a key enzyme-catalyzed hydrolysis reaction afforded the water-soluble deprotected α-anomer while leaving the β-anomer completely untouched.
Figures
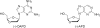
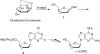

Similar articles
-
Pharmacokinetics of (-)-beta-D-dioxolane guanine and prodrug (-)-beta-D-2,6-diaminopurine dioxolane in rats and monkeys.AIDS Res Hum Retroviruses. 1999 Dec 10;15(18):1625-30. doi: 10.1089/088922299309667. AIDS Res Hum Retroviruses. 1999. PMID: 10606085
-
The use of beta-D-2,6-diaminopurine dioxolane with or without mycophenolate mofetil in drug-resistant HIV infection.AIDS. 2007 Oct 1;21(15):2025-32. doi: 10.1097/QAD.0b013e3282364381. AIDS. 2007. PMID: 17885292 Clinical Trial.
-
Synthesis and anti-HIV activity of (-)-beta-D-(2R,4R)-1,3-dioxolane-2,6-diamino purine (DAPD) (amdoxovir) and (-)-beta-D-(2R,4R)-1,3-dioxolane guanosine (DXG) prodrugs.Antiviral Res. 2007 Sep;75(3):198-209. doi: 10.1016/j.antiviral.2007.03.005. Epub 2007 Mar 30. Antiviral Res. 2007. PMID: 17532483 Free PMC article.
-
In vitro combination of amdoxovir and the inosine monophosphate dehydrogenase inhibitors mycophenolic acid and ribavirin demonstrates potent activity against wild-type and drug-resistant variants of human immunodeficiency virus type 1.Antimicrob Agents Chemother. 2004 Nov;48(11):4387-94. doi: 10.1128/AAC.48.11.4387-4394.2004. Antimicrob Agents Chemother. 2004. PMID: 15504868 Free PMC article.
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
References
-
- Global summary of the AIDS epidemic, database of WHO. 2010 http://www.who.int/hiv/data/2011_epi_core_en.png.
-
- Thompson MA, Kessler HA, Eron JJ, Jr., Jacobson JM, Adda N, Shen G, Zong J, Harris J, Moxham C, Rousseau FS. AIDS. 2005;19:1607. - PubMed
- Gripshover BM, Ribaudo H, Santana J, Gerber JG, Campbell TB, Hogg E, Jarocki B, Hammer SM, Kuritzkes DR, A5118 Team Antivir Ther. 2006;11:619. - PubMed
- Margolis DM, Mukherjee AL, Fletcher CV, Hogg E, Ogata-Arakaki D, Petersen T, Rusin D, Martinez A, Mellors JW. AIDS. 2007;21:2025. - PubMed
-
- Gu Z, Wainberg MA, Nguyen-Ba N, L'Heureux L, de Muys JM, Bowlin TL, Rando RF. Antimicrob Agents Chemother. 1999;43:2376. - PMC - PubMed
- Gu Z, Wainberg MA, Nguyen-Ba P, L'Heureux L, de Muys JM, Rando RF. Nucleosides Nucleotides. 1999;18:891. - PubMed
- Mewshaw JP, Myrick FT, Wakefield DA, Hooper BJ, Harris JL, McCreedy B, Borroto-Esoda K. J Acquir Immune Defic Syndr. 2002;29:11. - PubMed
-
- Murphy RL, Kivel NM, Zala C, Ochoa C, Tharnish P, Mathew J, Pascual ML, Schinazi RF. Antivir. Ther. 2010;15:185. - PMC - PubMed
- Hurwitz SJ, Asif G, Fromentin E, Tharnish PM, Schinazi RF. Antimicrob. Agents Chemother. 2010;54:1248. - PMC - PubMed
- Won SY, Kessler HA. In: Kucers' The Use of Antibiotics Sixth Edition. Grayson ML, editor. Vol 1. Hodder Arnold; 2010. p. 2627.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
