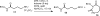Preparation of bifunctional isocyanate hydroxamate linkers: Synthesis of carbamate and urea tethered polyhydroxamic acid chelators
- PMID: 23162172
- PMCID: PMC3498463
- DOI: 10.1016/j.tetlet.2012.09.025
Preparation of bifunctional isocyanate hydroxamate linkers: Synthesis of carbamate and urea tethered polyhydroxamic acid chelators
Abstract
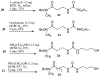
Two novel bifunctional N-methylhydroxamate-isocyanate linkers 20 and 21 were prepared in good yield and high purity from the corresponding amine salts using a biphasic reaction with phosgene. The facile ring opening reaction of N-Boc lactams using the anion of O-benzylhydroxylamine gave the protected amino hydroxamates 6a and 6c in good yields. The selective methylation of the hydroxamate nitrogen in the presence of the N-Boc group in these intermediates could be readily accomplished. The utility of the linkers was clearly demonstrated by the synthesis of the carbamate-tethered trishydroxamic acid 27 and the urea-tethered 29.
Figures
References
-
- Ji C, Juarez-Hernandez RE, Miller MJ. Future Med Chem. 2012;4:297. - PMC - PubMed
- Kovacic P, Edwards CL. Journal of Receptors and Signal Transduction. 2011;31(1):10. - PubMed
- Frederick RE, Mayfield JA, DuBois JL. Biometals. 2009;22:583. - PMC - PubMed
- Wencewicz TA, Möllmann U, Long TE, Miller MJ. Biometals. 2009;22:633. - PMC - PubMed
- Crisponi G, Remelli M. Coord Chem Rev. 2008;252:1225.
- Muri EMF, Nieto MJ, Williamson JS. Medicinal Chemistry Reviews-Online. 2004;1:385.
Grants and funding
LinkOut - more resources
Full Text Sources