Inhibitory control of hippocampal inhibitory neurons
- PMID: 23162426
- PMCID: PMC3496901
- DOI: 10.3389/fnins.2012.00165
Inhibitory control of hippocampal inhibitory neurons
Abstract
Information processing within neuronal networks is determined by a dynamic partnership between principal neurons and local circuit inhibitory interneurons. The population of GABAergic interneurons is extremely heterogeneous and comprises, in many brain regions, cells with divergent morphological and physiological properties, distinct molecular expression profiles, and highly specialized functions. GABAergic interneurons have been studied extensively during the past two decades, especially in the hippocampus, which is a relatively simple cortical structure. Different types of hippocampal inhibitory interneurons control spike initiation [e.g., axo-axonic and basket cells (BCs)] and synaptic integration (e.g., bistratified and oriens-lacunosum moleculare interneurons) within pyramidal neurons and synchronize local network activity, providing a means for functional segregation of neuronal ensembles and proper routing of hippocampal information. Thus, it is thought that, at least in the hippocampus, GABAergic inhibitory interneurons represent critical regulating elements at all stages of information processing, from synaptic integration and spike generation to large-scale network activity. However, this raises an important question: if inhibitory interneurons are fundamental for network computations, what are the mechanisms that control the activity of the interneurons themselves? Given the essential role of synaptic inhibition in the regulation of neuronal activity, it would be logical to expect that specific inhibitory mechanisms have evolved to control the operation of interneurons. Here, we review the mechanisms of synaptic inhibition of interneurons and discuss their role in the operation of hippocampal inhibitory circuits.
Keywords: GABA; hippocampus; inhibition; interneuron-specific interneuron; synapse.
Figures
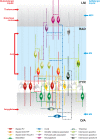
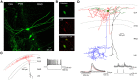
References
LinkOut - more resources
Full Text Sources
Miscellaneous

