Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity
- PMID: 23162431
- PMCID: PMC3499765
- DOI: 10.3389/fncel.2012.00054
Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity
Abstract
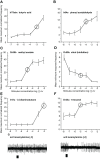
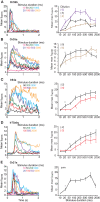
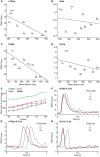
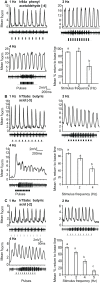
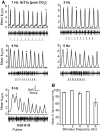
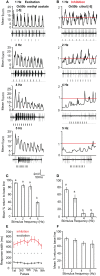
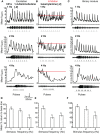
Insect olfactory sensory neurons (OSN) express a diverse array of receptors from different protein families, i.e. ionotropic receptors (IR), gustatory receptors (GR) and odorant receptors (OR). It is well known that insects are exposed to a plethora of odor molecules that vary widely in both space and time under turbulent natural conditions. In addition to divergent ligand specificities, these different receptors might also provide an increased range of temporal dynamics and sensitivities for the olfactory system. To test this, we challenged different Drosophila OSNs with both varying stimulus durations (10-2000 ms), and repeated stimulus pulses of key ligands at various frequencies (1-10 Hz). Our results show that OR-expressing OSNs responded faster and with higher sensitivity to short stimulations as compared to IR- and Gr21a-expressing OSNs. In addition, OR-expressing OSNs could respond to repeated stimulations of excitatory ligands up to 5 Hz, while IR-expressing OSNs required ~5x longer stimulations and/or higher concentrations to respond to similar stimulus durations and frequencies. Nevertheless, IR-expressing OSNs did not exhibit adaptation to longer stimulations, unlike OR- and Gr21a-OSNs. Both OR- and IR-expressing OSNs were also unable to resolve repeated pulses of inhibitory ligands as fast as excitatory ligands. These differences were independent of the peri-receptor environment in which the receptors were expressed and suggest that the receptor expressed by a given OSN affects both its sensitivity and its response to transient, intermittent chemical stimuli. OR-expressing OSNs are better at resolving low dose, intermittent stimuli, while IR-expressing OSNs respond more accurately to long-lasting odor pulses. This diversity increases the capacity of the insect olfactory system to respond to the diverse spatiotemporal signals in the natural environment.
Keywords: ionotropic receptors; odorant receptors; pulse resolution; single sensillum recording.
Figures
References
-
- Baker T. C., Vickers N. J. (1997). Pheromone-mediated flight in moths, In Pheromone Research: New Directions. ed Cardé R. T. (Minks AK. New York: Chapman and Hall; ), 248–264
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

