Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair?
- PMID: 23162470
- PMCID: PMC3493883
- DOI: 10.3389/fphys.2012.00424
Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair?
Abstract
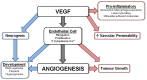
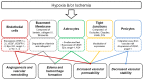
As clinicians attempt to understand the underlying reasons for the vulnerability of different regions of the developing brain to injury, it is apparent that little is known as to how hypoxia-ischemia may affect the cerebrovasculature in the developing infant. Most of the research investigating the pathogenesis of perinatal brain injury following hypoxia-ischemia has focused on excitotoxicity, oxidative stress and an inflammatory response, with the response of the developing cerebrovasculature receiving less attention. This is surprising as the presentation of devastating and permanent injury such as germinal matrix-intraventricular haemorrhage (GM-IVH) and perinatal stroke are of vascular origin, and the origin of periventricular leukomalacia (PVL) may also arise from poor perfusion of the white matter. This highlights that cerebrovasculature injury following hypoxia could primarily be responsible for the injury seen in the brain of many infants diagnosed with hypoxic-ischemic encephalopathy (HIE). Interestingly the highly dynamic nature of the cerebral blood vessels in the fetus, and the fluctuations of cerebral blood flow and metabolic demand that occur following hypoxia suggest that the response of blood vessels could explain both regional protection and vulnerability in the developing brain. However, research into how blood vessels respond following hypoxia-ischemia have mostly been conducted in adult models of ischemia or stroke, further highlighting the need to investigate how the developing cerebrovasculature responds and the possible contribution to perinatal brain injury following hypoxia. This review discusses the current concepts on the pathogenesis of perinatal brain injury, the development of the fetal cerebrovasculature and the blood brain barrier (BBB), and key mediators involved with the response of cerebral blood vessels to hypoxia.
Keywords: angiogenesis; blood-brain barrier; cerebral blood vessels; hemorrhage; hypoxia.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

