Sculpting humoral immunity through dengue vaccination to enhance protective immunity
- PMID: 23162552
- PMCID: PMC3492872
- DOI: 10.3389/fimmu.2012.00334
Sculpting humoral immunity through dengue vaccination to enhance protective immunity
Abstract
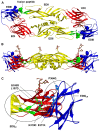
Dengue viruses (DENV) are the most important mosquito transmitted viral pathogens infecting humans. DENV infection produces a spectrum of disease, most commonly causing a self-limiting flu-like illness known as dengue fever; yet with increased frequency, manifesting as life-threatening dengue hemorrhagic fever (DHF). Waning cross-protective immunity from any of the four dengue serotypes may enhance subsequent infection with another heterologous serotype to increase the probability of DHF. Decades of effort to develop dengue vaccines are reaching the finishing line with multiple candidates in clinical trials. Nevertheless, concerns remain that imbalanced immunity, due to the prolonged prime-boost schedules currently used in clinical trials, could leave some vaccinees temporarily unprotected or with increased susceptibility to enhanced disease. Here we develop a DENV serotype 1 (DENV-1) DNA vaccine with the immunodominant cross-reactive B cell epitopes associated with immune enhancement removed. We compare wild-type (WT) with this cross-reactivity reduced (CRR) vaccine and demonstrate that both vaccines are equally protective against lethal homologous DENV-1 challenge. Under conditions mimicking natural exposure prior to acquiring protective immunity, WT vaccinated mice enhanced a normally sub-lethal heterologous DENV-2 infection resulting in DHF-like disease and 95% mortality in AG129 mice. However, CRR vaccinated mice exhibited redirected serotype-specific and protective immunity, and significantly reduced morbidity and mortality not differing from naїve mice. Thus, we demonstrate in an in vivo DENV disease model, that non-protective vaccine-induced immunity can prime vaccinees for enhanced DHF-like disease and that CRR DNA immunization significantly reduces this potential vaccine safety concern. The sculpting of immune memory by the modified vaccine and resulting redirection of humoral immunity provide insight into DENV vaccine-induced immune responses.
Keywords: DNA vaccines; antibody-dependent enhancement; dengue; dengue hemorrhagic fever; dengue vaccines; immune refocusing; original antigenic sin; vaccine safety.
Figures

Similar articles
-
Mapping the Human Memory B Cell and Serum Neutralizing Antibody Responses to Dengue Virus Serotype 4 Infection and Vaccination.J Virol. 2017 Feb 14;91(5):e02041-16. doi: 10.1128/JVI.02041-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28031369 Free PMC article.
-
Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection.EBioMedicine. 2016 Nov;13:284-293. doi: 10.1016/j.ebiom.2016.10.006. Epub 2016 Oct 7. EBioMedicine. 2016. PMID: 27746192 Free PMC article.
-
Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens.PLoS One. 2009;4(4):e4991. doi: 10.1371/journal.pone.0004991. Epub 2009 Apr 1. PLoS One. 2009. PMID: 19337372 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Challenges for the formulation of a universal vaccine against dengue.Exp Biol Med (Maywood). 2013 May;238(5):566-78. doi: 10.1177/1535370212473703. Exp Biol Med (Maywood). 2013. PMID: 23856907 Review.
Cited by
-
Complexity of Human Antibody Response to Dengue Virus: Implication for Vaccine Development.Front Microbiol. 2017 Jul 20;8:1372. doi: 10.3389/fmicb.2017.01372. eCollection 2017. Front Microbiol. 2017. PMID: 28775720 Free PMC article. Review.
-
Japanese Encephalitis DNA Vaccines with Epitope Modification Reduce the Induction of Cross-Reactive Antibodies against Dengue Virus and Antibody-Dependent Enhancement of Dengue Virus Infection.Vaccines (Basel). 2022 Aug 28;10(9):1411. doi: 10.3390/vaccines10091411. Vaccines (Basel). 2022. PMID: 36146489 Free PMC article.
-
Comprehensive Evaluation of Differential Serodiagnosis between Zika and Dengue Viral Infections.J Clin Microbiol. 2019 Feb 27;57(3):e01506-18. doi: 10.1128/JCM.01506-18. Print 2019 Mar. J Clin Microbiol. 2019. PMID: 30541932 Free PMC article.
-
Zika virus-like particle vaccine fusion loop mutation increases production yield but fails to protect AG129 mice against Zika virus challenge.PLoS Negl Trop Dis. 2022 Jul 6;16(7):e0010588. doi: 10.1371/journal.pntd.0010588. eCollection 2022 Jul. PLoS Negl Trop Dis. 2022. PMID: 35793354 Free PMC article.
-
Virus-like particle secretion and genotype-dependent immunogenicity of dengue virus serotype 2 DNA vaccine.J Virol. 2014 Sep;88(18):10813-30. doi: 10.1128/JVI.00810-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008922 Free PMC article.
References
-
- Brehm M. A., Pinto A. K., Daniels K. A., Schneck J. P., Welsh R. M., Selin L. K. (2002). T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3 627–634 - PubMed
-
- Chang G. J., Davis B. S., Hunt A. R., Holmes D. A., Kuno G. (2001). Flavivirus DNA vaccines: current status and potential. Ann. N. Y. Acad. Sci. 951 272–285 - PubMed
-
- Chang G. J., Davis B. S., Stringfield C., Lutz C. (2007). Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine 25 2325–2330 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

