Human NK cell lytic granules and regulation of their exocytosis
- PMID: 23162553
- PMCID: PMC3494098
- DOI: 10.3389/fimmu.2012.00335
Human NK cell lytic granules and regulation of their exocytosis
Abstract
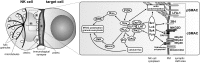
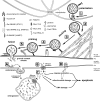
Natural killer (NK) cells form a subset of lymphocytes that play a key role in immuno-surveillance and host defense against cancer and viral infections. They recognize stressed cells through a variety of germline-encoded activating cell surface receptors and utilize their cytotoxic ability to eliminate abnormal cells. Killing of target cells is a complex, multi-stage process that concludes in the directed secretion of lytic granules, containing perforin and granzymes, at the immunological synapse. Upon delivery to a target cell, perforin mediates generation of pores in membranes of target cells, allowing granzymes to access target cell cytoplasm and induce apoptosis. Therefore, lytic granules of NK cells are indispensable for normal NK cell cytolytic function. Indeed, defects in lytic granule secretion lead or are related to serious and often fatal diseases, such as familial hemophagocytic lymphohistiocytosis (FHL) type 2-5 or Griscelli syndrome type 2. A number of reports highlight the role of several proteins involved in lytic granule release and NK cell-mediated killing of tumor cells. This review focuses on lytic granules of human NK cells and the advancements in understanding the mechanisms controlling their exocytosis.
Keywords: NK cells; cytotoxic lymphocytes; cytotoxicity; exocytosis; lysosomes; lytic granules.
Figures
References
-
- Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., et al. (2000). The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 - PubMed
-
- Almeida C. R., Davis D. M. (2006). Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J. Immunol. 177, 6904–6910 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

