Evolutionary Adaptations of Plant AGC Kinases: From Light Signaling to Cell Polarity Regulation
- PMID: 23162562
- PMCID: PMC3499706
- DOI: 10.3389/fpls.2012.00250
Evolutionary Adaptations of Plant AGC Kinases: From Light Signaling to Cell Polarity Regulation
Abstract
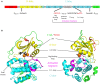
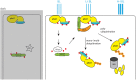
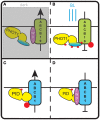
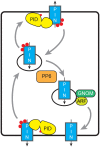
Signaling and trafficking over membranes involves a plethora of transmembrane proteins that control the flow of compounds or relay specific signaling events. Next to external cues, internal stimuli can modify the activity or abundance of these proteins at the plasma membrane (PM). One such regulatory mechanism is protein phosphorylation by membrane-associated kinases, several of which are AGC kinases. The AGC kinase family is one of seven kinase families that are conserved in all eukaryotic genomes. In plants evolutionary adaptations introduced specific structural changes within the AGC kinases that most likely allow modulation of kinase activity by external stimuli (e.g., light). Starting from the well-defined structural basis common to all AGC kinases we review the current knowledge on the structure-function relationship in plant AGC kinases. Nine of the 39 Arabidopsis AGC kinases have now been shown to be involved in the regulation of auxin transport. In particular, AGC kinase-mediated phosphorylation of the auxin transporters ABCB1 and ABCB19 has been shown to regulate their activity, while auxin transporters of the PIN family are located to different positions at the PM depending on their phosphorylation status, which is a result of counteracting AGC kinase and PP6 phosphatase activities. We therefore focus on regulation of AGC kinase activity in this context. Identified structural adaptations of the involved AGC kinases may provide new insight into AGC kinase functionality and demonstrate their position as central hubs in the cellular network controlling plant development and growth.
Keywords: AGC kinase; Arabidopsis thaliana; PIN polarity; evolution; kinase structure; phototropic growth.
Figures
References
-
- Bai F., Watson J. C., Walling J., Weeden N., Santner A. A., Demason D. A. (2005). Molecular characterization and expression of PsPK2, a PINOID-like gene from pea (Pisum sativum). Plant Sci. 168, 1281–129110.1016/j.plantsci.2005.01.005 - DOI
-
- Balendran A., Biondi R. M., Cheung P. C., Casamayor A., Deak M., Alessi D. R. (2000). A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK1. J. Biol. Chem. 275, 20806–2081310.1074/jbc.M000421200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

