Nedaplatin and irinotecan combination therapy is equally effective and less toxic than cisplatin and irinotecan for patients with primary clear cell adenocarcinoma of the ovary and recurrent ovarian carcinoma
- PMID: 23162643
- PMCID: PMC3499502
- DOI: 10.3892/ol.2012.853
Nedaplatin and irinotecan combination therapy is equally effective and less toxic than cisplatin and irinotecan for patients with primary clear cell adenocarcinoma of the ovary and recurrent ovarian carcinoma
Abstract
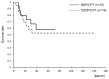
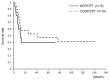
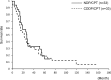
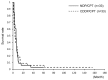
This study retrospectively compared nedaplatin and irinotecan hydrochloride (NDP/CPT) combination therapy with cisplatin and irinotecan hydrochloride therapy (CDDP/CPT) for efficacy and adverse events in the treatment of clear cell adenocarcinoma of the ovary (CCC) and recurrent ovarian carcinoma. A total of 115 patients were included in the present study. NDP/CPT was administered intravenously every 4 weeks (NDP, 60 mg/m(2) on day 1; CPT, 50 mg/m(2) on days 1, 8 and 15). CDDP/CPT was also administered intravenously (CDDP, 60 mg/m(2) on day 1; CPT, 60 mg/m(2) on days 1, 8 and 15). Patients with primary CCC were treated with NDP/CPT in 29 cases and CDDP/CPT in 20 cases. Patients with recurrent ovarian carcinoma were treated with NDP/CPT and CDDP/CPT in 33 cases each. No significant difference was observed in the 5-year overall survival (OS)/progression-free survival (PFS) of patients with primary CCC, with the exception of those patients with stages Ia and Ic(b) who underwent NDP/CPT and CDDP/CPT treatments (OS: 58%, PFS: 40% and OS: 53% and PFS: 47%, respectively). No significant differences were found in the response rates to NDP/CPT and CDDP/CPT in patients with recurrent ovarian carcinoma (27 and 18%, respectively). Similarly, there were no significant differences in the 5-year OS and PFS of patients with recurrent ovarian carcinoma treated with NDP/CPT or CDDP/CPT (OS: 15%, PFS: 3% and OS: 18%, PFS: 6%, respectively). In terms of the hematological toxicity of grade 3 or above and non-hematological toxicity of grade 2 or above in patients treated with NDP/CPT and CDDP/CPT, respectively, neutropenia was 23 and 56%; anemia, 1, and 20%; thrombocytopenia, 0 and 5%; nausea, 20 and 52%; diarrhea, 14 and 25%; and fever, 2 and 11%. Accordingly, NDP/CPT indicated mild toxicity, and was therefore equally effective and less toxic than CDDP/CPT in the treatment of primary CCC and recurrent ovarian carcinoma.
Figures
Similar articles
-
[Combination of irinotecan hydrochloride (CPT-11) and cisplatin as a new regimen for patients with advanced ovarian cancer].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Sep;48(9):827-34. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8841050 Clinical Trial. Japanese.
-
Combination of irinotecan (CPT-11) and nedaplatin (NDP) for recurrent patients with uterine cervical cancer.Int J Clin Oncol. 2013 Dec;18(6):1102-6. doi: 10.1007/s10147-012-0487-4. Epub 2012 Oct 25. Int J Clin Oncol. 2013. PMID: 23095879
-
Retrospective comparative study of irinotecan and pegylated liposomal doxorubicin for platinum-resistant or -refractory epithelial ovarian and primary peritoneal carcinoma.Arch Gynecol Obstet. 2014 Nov;290(5):979-84. doi: 10.1007/s00404-014-3268-7. Epub 2014 May 6. Arch Gynecol Obstet. 2014. PMID: 24798935
-
Efficacy of combination chemotherapy using irinotecan and nedaplatin for patients with recurrent and refractory endometrial carcinomas: preliminary analysis and literature review.Cancer Chemother Pharmacol. 2018 Jan;81(1):111-117. doi: 10.1007/s00280-017-3454-y. Epub 2017 Nov 9. Cancer Chemother Pharmacol. 2018. PMID: 29124328 Review.
-
[Promising new drugs for gynecological cancer].Gan To Kagaku Ryoho. 1997 Oct;24(13):1932-7. Gan To Kagaku Ryoho. 1997. PMID: 9350238 Review. Japanese.
Cited by
-
Risk Factors for Septic Shock After Irinotecan-Containing Chemotherapy: An Exploratory Case-Control Study.Drugs R D. 2022 Dec;22(4):263-269. doi: 10.1007/s40268-022-00399-y. Epub 2022 Aug 20. Drugs R D. 2022. PMID: 35987938 Free PMC article.
-
Impact of the number of removed lymph nodes on recurrence-free survival in stage I ovarian clear cell carcinoma.Int J Clin Oncol. 2018 Oct;23(5):930-935. doi: 10.1007/s10147-018-1280-9. Epub 2018 Apr 20. Int J Clin Oncol. 2018. PMID: 29679177
References
-
- Serov SF, Scully RE, Sobin LH, editors. International Histologic Classification of Tumors, No 9 Histological Typing of Ovarian Tumors. World Health Organization; Geneva: 1973.
-
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 6th Annual Report on Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S161–S192. - PubMed
-
- Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. - PubMed
-
- Du Bois A, Herrstedt J, Hardy-Bessard AC, et al. Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. J Clin Oncol. 2010;28:4162–4169. - PubMed
-
- Utsunomiya H, Akahira J, Tanno S, et al. Paclitaxel-platinum combination chemotherapy for advanced or recurrent ovarian clear cell adenocarcinoma: a multicenter trial. Int J Gynecol Cancer. 2006;16:52–56. - PubMed
LinkOut - more resources
Full Text Sources
