Cell type-specific activation of AKT and ERK signaling pathways by small negatively-charged magnetic nanoparticles
- PMID: 23162692
- PMCID: PMC3499776
- DOI: 10.1038/srep00868
Cell type-specific activation of AKT and ERK signaling pathways by small negatively-charged magnetic nanoparticles
Abstract
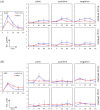
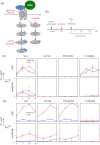
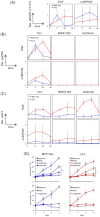
The interaction of nanoparticles (NPs) with living organisms has become a focus of public and scientific debate due to their potential wide applications in biomedicine, but also because of unwanted side effects. Here, we show that superparamagnetic iron oxide NPs (SPIONs) with different surface coatings can differentially affect signal transduction pathways. Using isogenic pairs of breast and colon derived cell lines we found that the stimulation of ERK and AKT signaling pathways by SPIONs is selectively dependent on the cell type and SPION type. In general, cells with Ras mutations respond better than their non-mutant counterparts. Small negatively charged SPIONs (snSPIONs) activated ERK to a similar extent as epidermal growth factor (EGF), and used the same upstream signaling components including activation of the EGF receptor. Importantly, snSPIONs stimulated the proliferation of Ras transformed breast epithelial cells as efficiently as EGF suggesting that NPs can mimic physiological growth factors.
Figures


References
-
- Grecco H. E., Schmick M. & Bastiaens P. I. H. Signaling from the Living Plasma Membrane. Cell 144, 897–909 (2011). - PubMed
-
- Eccles S. A. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol 55, 685–696 (2011). - PubMed
-
- Citri A. & Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7, 505–516 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

