Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers
- PMID: 23162712
- PMCID: PMC3493240
- DOI: 10.1364/BOE.3.002733
Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers
Abstract
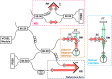
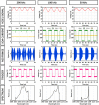
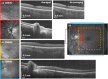
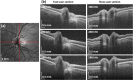
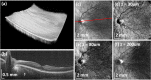
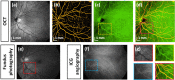
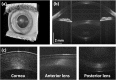
We demonstrate swept source OCT utilizing vertical-cavity surface emitting laser (VCSEL) technology for in vivo high speed retinal, anterior segment and full eye imaging. The MEMS tunable VCSEL enables long coherence length, adjustable spectral sweep range and adjustable high sweeping rate (50-580 kHz axial scan rate). These features enable integration of multiple ophthalmic applications into one instrument. The operating modes of the device include: ultrahigh speed, high resolution retinal imaging (up to 580 kHz); high speed, long depth range anterior segment imaging (100 kHz) and ultralong range full eye imaging (50 kHz). High speed imaging enables wide-field retinal scanning, while increased light penetration at 1060 nm enables visualization of choroidal vasculature. Comprehensive volumetric data sets of the anterior segment from the cornea to posterior crystalline lens surface are also shown. The adjustable VCSEL sweep range and rate make it possible to achieve an extremely long imaging depth range of ~50 mm, and to demonstrate the first in vivo 3D OCT imaging spanning the entire eye for non-contact measurement of intraocular distances including axial eye length. Swept source OCT with VCSEL technology may be attractive for next generation integrated ophthalmic OCT instruments.
Keywords: (110.4500) Optical coherence tomography; (120.4640) Optical instruments; (140.3600) Lasers, tunable; (170.4460) Ophthalmic optics and devices; (170.4470) Ophthalmology.
Figures




References
-
- J. Schuman, C. A. Puliafito, and J. Fujimoto, eds., Optical Coherence Tomography of Ocular Diseases, 2nd ed. (Slack Inc., Thorofare, 2004), pp. 1–250.
-
- Izatt J. A., Hee M. R., Swanson E. A., Lin C. P., Huang D., Schuman J. S., Puliafito C. A., Fujimoto J. G., “Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography,” Arch. Ophthalmol. 112(12), 1584–1589 (1994).10.1001/archopht.1994.01090240090031 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
