Automatic stent detection in intravascular OCT images using bagged decision trees
- PMID: 23162720
- PMCID: PMC3493217
- DOI: 10.1364/BOE.3.002809
Automatic stent detection in intravascular OCT images using bagged decision trees
Abstract
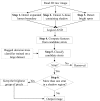
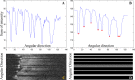
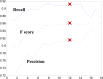
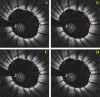
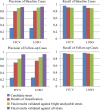
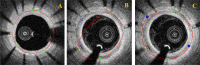
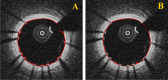
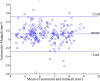
Intravascular optical coherence tomography (iOCT) is being used to assess viability of new coronary artery stent designs. We developed a highly automated method for detecting stent struts and measuring tissue coverage. We trained a bagged decision trees classifier to classify candidate struts using features extracted from the images. With 12 best features identified by forward selection, recall (precision) were 90%-94% (85%-90%). Including struts deemed insufficiently bright for manual analysis, precision improved to 94%. Strut detection statistics approached variability of manual analysis. Differences between manual and automatic area measurements were 0.12 ± 0.20 mm(2) and 0.11 ± 0.20 mm(2) for stent and tissue areas, respectively. With proposed algorithms, analyst time per stent should significantly reduce from the 6-16 hours now required.
Keywords: (100.0100) Image processing; (110.4500) Optical coherence tomography.
Figures



References
-
- Beijk M. A., Klomp M., Verouden N. J., van Geloven N., Koch K. T., Henriques J. P., Baan J., Vis M. M., Scheunhage E., Piek J. J., Tijssen J. G., de Winter R. J., “Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study,” Eur. Heart J. 31(9), 1055–1064 (2010).10.1093/eurheartj/ehp476 - DOI - PMC - PubMed
-
- Granada J. F., Inami S., Aboodi M. S., Tellez A., Milewski K., Wallace-Bradley D., Parker S., Rowland S., Nakazawa G., Vorpahl M., Kolodgie F. D., Kaluza G. L., Leon M. B., Virmani R., “Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix,” Circ Cardiovasc Interv 3(3), 257–266 (2010).10.1161/CIRCINTERVENTIONS.109.919936 - DOI - PubMed
-
- Guagliumi G., Musumeci G., Sirbu V., Bezerra H. G., Suzuki N., Fiocca L., Matiashvili A., Lortkipanidze N., Trivisonno A., Valsecchi O., Biondi-Zoccai G., Costa M. A., ODESSA Trial Investigators , “Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents,” JACC Cardiovasc. Interv. 3(5), 531–539 (2010).10.1016/j.jcin.2010.02.008 - DOI - PubMed
-
- Guagliumi G., Sirbu V., Musumeci G., Bezerra H. G., Aprile A., Kyono H., Fiocca L., Matiashvili A., Lortkipanidze N., Vassileva A., Popma J. J., Allocco D. J., Dawkins K. D., Valsecchi O., Costa M. A., “Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI),” Circ Cardiovasc Interv 3(4), 367–375 (2010).10.1161/CIRCINTERVENTIONS.110.950154 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
