High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes
- PMID: 23162750
- PMCID: PMC3489738
- DOI: 10.4161/onci.20492
High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes
Abstract
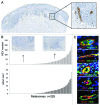
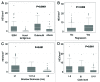
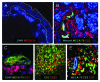
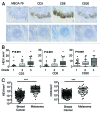
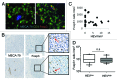
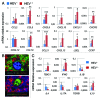
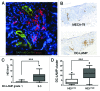
The presence of tumor-infiltrating lymphocytes (TILs) is a strong prognostic parameter for local dissemination and overall survival in melanoma. Lymphocyte migration from blood into peripheral tissues is mainly regulated by vascular endothelium. However, the blood vessels and mechanisms governing the recruitment of TILs in melanoma tumors remain poorly understood. Here, we show that high endothelial venules (HEVs), specialized blood vessels for lymphocyte extravasation into lymphoid tissues, are frequently found in melanoma tumors and are associated with high levels of lymphocyte infiltration. The analysis of 225 primary melanomas revealed that lymphocytes specifically infiltrated HEV-rich areas of melanoma tumors and that the density of MECA-79+ HEVs was variable among patients and strongly correlated with CD3+, CD8+ and CD20+ TIL densities. Inflammatory (CCL5, CXCL9, CXCL10 and CXCL11) and lymphoid (CCL21, CCL19 and CXCL13) chemokines as well as TH1 and naïve T-cell genes were overexpressed in melanoma samples with high densities of tumor HEVs. Mature dendritic cells (mDCs) were frequently found around tumor HEVs and densities of HEVs and DC-LAMP+ mDCs within tumor stroma were strongly correlated. DCs which maintain HEVs in lymph nodes, may thus also contribute to the regulation of HEVs in melanomas. Finally, we found significantly higher densities of tumor HEVs in melanomas with tumor regression, low Clark level of invasion and thin Breslow thickness (all p < 0.001). The strong association between tumor HEVs, TILs, mDCs and clinical parameters of melanoma, supports a critical role for HEVs in limiting malignant melanoma development through both naïve and effector T-lymphocyte recruitment and activation.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
