Mesenchymal stem cells as a novel vaccine platform
- PMID: 23162801
- PMCID: PMC3499769
- DOI: 10.3389/fcimb.2012.00140
Mesenchymal stem cells as a novel vaccine platform
Abstract
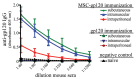
Vaccines are the most efficient and cost-effective means of preventing infectious disease. However, traditional vaccine approaches have thus far failed to provide protection against human immunodeficiency virus (HIV), tuberculosis, malaria, and many other diseases. New approaches to vaccine development are needed to address some of these intractable problems. In this report, we review the literature identifying stimulatory effects of mesenchymal stem cells (MSC) on immune responses and explore the potential for MSC as a novel, universal vaccination platform. MSC are unique bone marrow-derived multipotent progenitor cells that are presently being exploited as gene therapy vectors for a variety of conditions, including cancer and autoimmune diseases. Although MSC are predominantly known for anti-inflammatory properties during allogeneic MSC transplant, there is evidence that MSC can actually promote adaptive immunity under certain settings. MSC have also demonstrated some success in anti-cancer therapeutic vaccines and anti-microbial prophylactic vaccines, as we report, for the first time, the ability of modified MSC to express and secrete a viral antigen that stimulates antigen-specific antibody production in vivo. We hypothesize that the unique properties of modified MSC may enable MSC to serve as an unconventional but innovative, vaccine platform. Such a platform would be capable of expressing hundreds of proteins, thereby generating a broad array of epitopes with correct post-translational processing, mimicking natural infection. By stimulating immunity to a combination of epitopes, it may be possible to develop prophylactic and even therapeutic vaccines to tackle major health problems including those of non-microbial and microbial origin, including cancer, or an infectious disease like HIV, where traditional vaccination approaches have failed.
Keywords: MSC; adaptive immunity; antibodies; antigen delivery; vaccination.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

