Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial
- PMID: 23162860
- PMCID: PMC3517179
- DOI: 10.1001/jama.2012.14641
Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial
Erratum in
- JAMA. 2014 Aug 6;312(5):560
Abstract
Context: Multivitamin preparations are the most common dietary supplement, taken by at least one-third of all US adults. Observational studies have not provided evidence regarding associations of multivitamin use with total and site-specific cancer incidence or mortality.
Objective: To determine whether long-term multivitamin supplementation decreases the risk of total and site-specific cancer events among men.
Design, setting, and participants: A large-scale, randomized, double-blind, placebo controlled trial (Physicians" Health Study II) of 14 641 male US physicians initially aged 50 years or older (mean [SD] age, 64.3 [9.2] years), including 1312 men with a history of cancer at randomization, enrolled in a common multivitamin study that began in 1997 with treatment and follow-up through June 1, 2011.
Intervention: Daily multivitamin or placebo.
Main outcome measures: Total cancer (excluding nonmelanoma skin cancer), with prostate, colorectal, and other site-specific cancers among the secondary end points.
Results: During a median (interquartile range) follow-up of 11.2 (10.7-13.3) years, there were 2669 men with confirmed cancer, including 1373 cases of prostate cancer and 210 cases of colorectal cancer. Compared with placebo, men taking a daily multivitamin had a statistically significant reduction in the incidence of total cancer (multivitamin and placebo groups, 17.0 and 18.3 events, respectively, per 1000 person-years; hazard ratio [HR], 0.92; 95% CI, 0.86-0.998; P=.04). There was no significant effect of a daily multivitamin on prostate cancer (multivitamin and placebo groups, 9.1 and 9.2 events, respectively, per 1000 person-years; HR, 0.98; 95% CI, 0.88-1.09; P=.76), colorectal cancer (multivitamin and placebo groups, 1.2 and 1.4 events, respectively, per 1000 person-years; HR, 0.89; 95% CI, 0.68-1.17; P=.39), or other site-specific cancers. There was no significant difference in the risk of cancer mortality (multivitamin and placebo groups, 4.9 and 5.6 events, respectively, per 1000 person-years; HR, 0.88; 95% CI, 0.77-1.01; P=.07). Daily multivitamin use was associated with a reduction in total cancer among 1312 men with a baseline history of cancer (HR, 0.73; 95% CI, 0.56-0.96; P=.02), but this did not differ significantly from that among 13 329 men initially without cancer (HR, 0.94; 95% CI, 0.87-1.02; P=.15; P for interaction=.07). Conclusion In this large prevention trial of male physicians, daily multivitamin supplementation modestly but significantly reduced the risk of total cancer.
Trial registration: clinicaltrials.gov Identifier: NCT00270647.
Figures
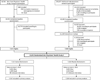
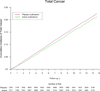



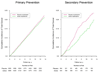
Comment in
-
Multiplicities in the assessment of multiple vitamins: is it too soon to tell men that vitamins prevent cancer?JAMA. 2012 Nov 14;308(18):1916-7. doi: 10.1001/jama.2012.53273. JAMA. 2012. PMID: 23150011 No abstract available.
-
Modest cancer prevention benefit with long-term multivitamin supplementation: Physicians' Health Study II results warrant cautious interpretation.Evid Based Med. 2013 Dec;18(6):214-5. doi: 10.1136/eb-2012-101188. Epub 2013 Feb 16. Evid Based Med. 2013. PMID: 23416420 No abstract available.
-
[Do vitamin supplements help fight cancer?].Dtsch Med Wochenschr. 2013 Feb;138(6):243. doi: 10.1055/s-0032-1331845. Dtsch Med Wochenschr. 2013. PMID: 23479791 German. No abstract available.
-
Multivitamins for cancer prevention in men.JAMA. 2013 Mar 13;309(10):980. doi: 10.1001/jama.2013.1188. JAMA. 2013. PMID: 23483158 No abstract available.
-
Multivitamins for cancer prevention in men.JAMA. 2013 Mar 13;309(10):980-1. doi: 10.1001/jama.2013.1181. JAMA. 2013. PMID: 23483159 No abstract available.
-
Multivitamins for cancer prevention in men-reply.JAMA. 2013 Mar 13;309(10):980-1. doi: 10.1001/jama.2013.1191. JAMA. 2013. PMID: 23483160 No abstract available.
-
Can daily multivitamin prevent cancer? Results from the Physicians' Health Study.Pol Arch Med Wewn. 2013;123(3):83-4. Pol Arch Med Wewn. 2013. PMID: 23535857 No abstract available.
References
-
- Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;(61):1–8. - PubMed
-
- Boffetta P, Couto E, Wichmann J, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2010;102(8):529–537. - PubMed
-
- Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–1584. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

