Cu(II)-catalyzed aerobic hydroperoxidation of Meldrum's acid derivatives and application in intramolecular oxidation: a conceptual blueprint for O2/H2 dihydroxylation
- PMID: 23163733
- PMCID: PMC3518688
- DOI: 10.1021/ol302848m
Cu(II)-catalyzed aerobic hydroperoxidation of Meldrum's acid derivatives and application in intramolecular oxidation: a conceptual blueprint for O2/H2 dihydroxylation
Abstract
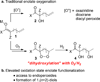
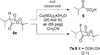
Aerobic hydroperoxidation of Meldrum's acid derivatives via a Cu(II)-catalyzed process is presented. The mild reaction conditions are tolerant to pendant unsaturation allowing the formation of endoperoxides via electrophilic activation. Cleavage of the O-O bond provides 1,n-diols with differentiation of the hydroxy groups.
Figures



Similar articles
-
Meldrum's acids and 5-alkylidene Meldrum's acids in catalytic carbon-carbon bond-forming processes.Acc Chem Res. 2010 Mar 16;43(3):440-54. doi: 10.1021/ar900229z. Acc Chem Res. 2010. PMID: 20000793
-
Scandium triflate-catalyzed nucleophilic additions to indolylmethyl Meldrum's acid derivatives via a gramine-type fragmentation: synthesis of substituted indolemethanes.J Org Chem. 2013 Oct 18;78(20):10534-40. doi: 10.1021/jo4017524. Epub 2013 Oct 8. J Org Chem. 2013. PMID: 24066671
-
Probing persistent intramolecular C-H...X (X = O, S, Br, Cl, and F) bonding in solution using benzyl Meldrum's acid derivatives.J Org Chem. 2009 Feb 6;74(3):1259-67. doi: 10.1021/jo802311w. J Org Chem. 2009. PMID: 19113820
-
One hundred years of Meldrum's acid: advances in the synthesis of pyridine and pyrimidine derivatives.Mol Divers. 2009 Nov;13(4):399-419. doi: 10.1007/s11030-009-9136-x. Epub 2009 Apr 21. Mol Divers. 2009. PMID: 19381852 Review.
-
Recent Advances in Copper Catalyzed Alcohol Oxidation in Homogeneous Medium.Molecules. 2020 Feb 9;25(3):748. doi: 10.3390/molecules25030748. Molecules. 2020. PMID: 32050493 Free PMC article. Review.
Cited by
-
Structural basis for endoperoxide-forming oxygenases.Beilstein J Org Chem. 2022 Jun 21;18:707-721. doi: 10.3762/bjoc.18.71. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35821691 Free PMC article. Review.
-
Donor-Acceptor Cyclopropanes: Activation Enabled by a Single, Vinylogous Acceptor.Angew Chem Int Ed Engl. 2023 Jan 2;62(1):e202214390. doi: 10.1002/anie.202214390. Epub 2022 Nov 30. Angew Chem Int Ed Engl. 2023. PMID: 36322458 Free PMC article.
-
Cyclization-endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst.Beilstein J Org Chem. 2014 Jun 3;10:1272-81. doi: 10.3762/bjoc.10.128. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24991279 Free PMC article.
References
-
- Smith AMR, Hii KK. Chem. Rev. 2011;111:1637–1656. - PubMed
-
-
Chen B-C, Zhou P, Davis FA, Ciganek E. Org. React. (N.Y.) 2003;62:1–356. (b) For direct hydroxylation of active methines using Pd(II)/O2 see: Chuang GJ, Wang W, Lee E, Ritter T. J. Am. Chem. Soc. 2011;133:1760–1762.
-
-
- Burns NZ, Baran PS, Hoffmann RW. Angew. Chem. Int. Ed. 2009;48:2854–2867. - PubMed
-
-
For recent reviews of Meldrum’s acid in organic synthesis: Dumas AM, Fillion E. Acc. Chem. Res. 2010;43:440–454. Ivanov AS. Chem. Soc. Rev. 2008;37:789–811.
-
-
- Keiser J, Utzinger J. Curr. Opin. Infect. Diseases. 2007;20:605–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

