Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and semipure lectin fractions on cancer cells
- PMID: 23163855
- PMCID: PMC3856472
- DOI: 10.1080/01635581.2012.722246
Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and semipure lectin fractions on cancer cells
Abstract
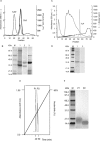
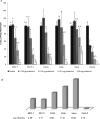
Some natural and synthetic protease inhibitors (PI), such as the Bowman-Birk PI from soybean, have anticancer effects. We previously purified and characterized a Bowman-Birk-type PI from Tepary bean (Phaseolus acutifolius) seeds (TBPI). A semipure protein fraction containing this inhibitor, when tested its in vitro effect on transformed cells, showed a differential cytotoxic effect, as well as an increase in cell attachment to culture dishes. In this article we report that lectins were responsible for the cytotoxic effect previously observed, exhibiting a differential, antiproliferative effect on nontransformed cells and on different lineages of cancer cells. Although the purified TBPI lacked cytotoxicity, it was found to be responsible for the increase in cell adhesion, decreasing culture dishes' extracellular matrix degradation, leading to a decrease of the in vitro cell invasion capacity. This effect coincided with the suppression of Matrix Metalloproteinase-9 activity. These results indicate that Tepary bean seeds contain at least 2 different groups of bioactive proteins with distinct effects on cancer cells.
Figures





Similar articles
-
The effects of a protease inhibitor fraction from tepary bean (Phaseolus acutifolius) on in vitro cell proliferation and cell adhesion of transformed cells.Toxicol In Vitro. 2002 Jun;16(3):229-33. doi: 10.1016/s0887-2333(02)00006-1. Toxicol In Vitro. 2002. PMID: 12020595
-
Tepary Bean (Phaseolus acutifolius) Lectins as Modulators of Intracellular Calcium Mobilization in Breast Cancer and Normal Breast Cells.Int J Mol Sci. 2025 Jan 26;26(3):1064. doi: 10.3390/ijms26031064. Int J Mol Sci. 2025. PMID: 39940827 Free PMC article.
-
Phaseolus acutifolius Lectin Fractions Exhibit Apoptotic Effects on Colon Cancer: Preclinical Studies Using Dimethilhydrazine or Azoxi-Methane as Cancer Induction Agents.Molecules. 2017 Oct 8;22(10):1670. doi: 10.3390/molecules22101670. Molecules. 2017. PMID: 28991196 Free PMC article.
-
Tepary bean (Phaseolus acutifolius).Methods Mol Biol. 2006;343:407-14. doi: 10.1385/1-59745-130-4:407. Methods Mol Biol. 2006. PMID: 16988363 Review.
-
[Characterization of the nutritional potential of tepari bean (Phaseolus acutifolius) grown in Mexico].Arch Latinoam Nutr. 1988 Dec;38(4):907-24. Arch Latinoam Nutr. 1988. PMID: 3154299 Review. Spanish.
Cited by
-
Tepary bean (Phaseolus acutifolius) lectin fraction provokes reversible adverse effects on rats' digestive tract.Toxicol Res (Camb). 2020 Oct 21;9(5):714-725. doi: 10.1093/toxres/tfaa062. eCollection 2020 Sep. Toxicol Res (Camb). 2020. PMID: 33178432 Free PMC article.
-
Cytotoxic and antiproliferative effect of tepary bean lectins on C33-A, MCF-7, SKNSH, and SW480 cell lines.Molecules. 2014 Jul 7;19(7):9610-27. doi: 10.3390/molecules19079610. Molecules. 2014. PMID: 25004071 Free PMC article.
-
Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay.Int J Mol Sci. 2021 Jan 21;22(3):1049. doi: 10.3390/ijms22031049. Int J Mol Sci. 2021. PMID: 33494324 Free PMC article.
-
Potential Role of Bioactive Proteins and Peptides Derived from Legumes towards Metabolic Syndrome.Nutrients. 2022 Dec 10;14(24):5271. doi: 10.3390/nu14245271. Nutrients. 2022. PMID: 36558429 Free PMC article. Review.
-
Effect of Induced Mechanical Leaf Damage on the Yield and Content of Bioactive Molecules in Leaves and Seeds of Tepary Beans (Phaseolus acutifolius).Plants (Basel). 2022 Dec 15;11(24):3538. doi: 10.3390/plants11243538. Plants (Basel). 2022. PMID: 36559649 Free PMC article.
References
-
- Yavelow J, Findlay HT, Kennedy AR, Troll W. Bowman-Birk soybean protease inhibitor as an antiarcinogen. Cancer Res. 1983;43(Suppl.):2454S–2459S. - PubMed
-
- Kennedy AR. Cancer prevention by protease inhibitors. Prev Med. 1993;22:796–811. - PubMed
-
- Gueven N, Dittman K, Mayer C, Rodemann HP. The radioprotective potential of the Bowman-Birk protease inhibitor is independent of its secondary structure. Cancer Lett. 1998;125:77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
