No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks
- PMID: 23163898
- PMCID: PMC3654567
- DOI: 10.1111/dom.12040
No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks
Abstract
Aims: The potential association of glucagon-like peptide receptor agonists (GLP-1RAs) with the development of pancreatitis or pancreatic malignancies in patients with diabetes has been suggested. This study evaluated the long-term effects of the GLP-1RA exenatide on pancreatic exocrine structure and function in the Zucker diabetic fatty (ZDF) rat model of type 2 diabetes.
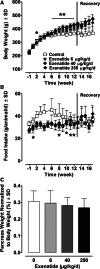
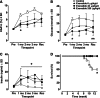
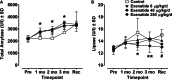
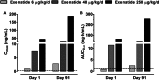
Methods: Rats received subcutaneous twice-daily injections of 0 (control), 6, 40 and 250 µg/kg/day exenatide for 3 months. Clinical signs, body and pancreas weight, food consumption, HbA1c, fasting serum amylase, lipase, glucose and insulin concentrations were evaluated during treatment and after a 28-day off-drug period to assess the reversibility of any observed effects. Morphometric analysis of pancreatic ductal cell proliferation and apoptosis were performed.
Results: Plasma exenatide concentrations were several-fold higher than therapeutic levels observed in humans. No exenatide-related effects were observed on clinical signs, lipase concentration, pancreatic weight, pancreatic histology, ductal cell proliferation or apoptosis. Exenatide improved animal survival, physical condition, glucose concentrations and HbA1c, decreased food intake, and increased serum insulin concentration. Total amylase concentrations, although within normal ranges, were slightly higher in exenatide-treated rats; following the off-drug period, total amylase concentrations were comparable in treated and untreated rats. Exenatide-related minimal-to-moderate islet hypertrophy was observed at doses ≥6 µg/kg/day, with dose-related increases in incidence and degree. These changes were still present after the off-drug period.
Conclusions: Chronic administration of exenatide in ZDF rats resulted in the expected metabolic benefits and improved animal survival, with no adverse effects noted on pancreatic exocrine structure and function.
© 2012 Blackwell Publishing Ltd.
Figures

References
-
- Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2007;9:23–31. - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. - PubMed
-
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. - PubMed
-
- Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29:471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

