Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy
- PMID: 23163975
- PMCID: PMC6496525
- DOI: 10.1111/cpr.12002
Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy
Abstract
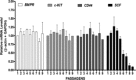
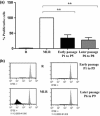
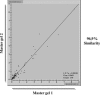
Ex vivo expansion and manipulation of human mesenchymal stem cells are important approaches to immunoregulatory and regenerative cell therapies. Although these cells show great potential for use, issues relating to their overall nature emerge as problems in the field. The need for extensive cell quantity amplification in vitro to obtain sufficient cell numbers for use, poses a risk of accumulating genetic and epigenetic abnormalities that could lead to sporadic malignant cell transformation. In this study, we have examined human mesenchymal stem cells derived from bone marrow, over extended culture time, using cytogenetic analyses, mixed lymphocyte reactions, proteomics and gene expression assays to determine whether the cultures would retain their potential for use in subsequent passages. Results indicate that in vitro cultures of these cells demonstrated chromosome variability after passage 4, but their immunomodulatory functions and differentiation capacity were maintained. At the molecular level, changes were observed from passage 5 on, indicating initiation of differentiation. Together, these results lead to the hypothesis that human mesenchymal stem cells cultures can be used successfully in cell therapy up to passage 4. However, use of cells from higher passages would have to be analysed case by case.
© 2012 Blackwell Publishing Ltd.
Figures



Similar articles
-
Chromosomal stability of mesenchymal stromal cells during in vitro culture.Cytotherapy. 2016 Mar;18(3):336-43. doi: 10.1016/j.jcyt.2015.11.017. Epub 2016 Jan 15. Cytotherapy. 2016. PMID: 26780865 Free PMC article.
-
Chromosomal variability of human mesenchymal stem cells cultured under hypoxic conditions.J Cell Mol Med. 2012 Jan;16(1):72-82. doi: 10.1111/j.1582-4934.2011.01303.x. J Cell Mol Med. 2012. PMID: 21418515 Free PMC article.
-
Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages.Haematologica. 2010 Jan;95(1):47-56. doi: 10.3324/haematol.2009.008524. Epub 2009 Aug 27. Haematologica. 2010. PMID: 19713224 Free PMC article.
-
Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion.Biomaterials. 2005 Nov;26(31):6167-75. doi: 10.1016/j.biomaterials.2005.03.024. Biomaterials. 2005. PMID: 15913765
-
From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera?Int J Dev Biol. 2008;52(8):1023-32. doi: 10.1387/ijdb.082725gt. Int J Dev Biol. 2008. PMID: 18956335 Review.
Cited by
-
Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model.Biomed Res Int. 2015;2015:527926. doi: 10.1155/2015/527926. Epub 2015 Feb 23. Biomed Res Int. 2015. PMID: 25802852 Free PMC article.
-
Therapeutic Potential of Autologous Adipose-Derived Stem Cells for the Treatment of Liver Disease.Int J Mol Sci. 2018 Dec 15;19(12):4064. doi: 10.3390/ijms19124064. Int J Mol Sci. 2018. PMID: 30558283 Free PMC article.
-
Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation.Cell Death Dis. 2013 Dec 5;4(12):e950. doi: 10.1038/cddis.2013.480. Cell Death Dis. 2013. PMID: 24309937 Free PMC article.
-
The Effect of Culture on Human Bone Marrow Mesenchymal Stem Cells: Focus on DNA Methylation Profiles.Stem Cells Int. 2016;2016:5656701. doi: 10.1155/2016/5656701. Epub 2016 Jan 6. Stem Cells Int. 2016. PMID: 26880970 Free PMC article.
-
Mesenchymal stem cells and mesenchymal stem cell-derived exosomes: attractive therapeutic approaches for female reproductive dysfunction.Mol Biol Rep. 2024 Nov 22;52(1):10. doi: 10.1007/s11033-024-10106-6. Mol Biol Rep. 2024. PMID: 39576370 Review.
References
-
- Kassem M, Abdallah BM (2008) Human bone‐marrow‐derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 331, 157–163. - PubMed
-
- Bolland BJ, Tilley S, New AM, Dunlop DG, Oreffo RO (2007) Adult mesenchymal stem cells and impaction grafting: a new clinical paradigm shift. Expert Rev. Med. Devices 4, 393–404. - PubMed
-
- Abdel‐Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA et al (2007) Adult bone marrow‐derived cells for cardiac repair: a systematic review and meta‐analysis. Arch. Intern. Med. 167, 989–997. - PubMed
-
- Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H et al (2006) Mesenchymal stem cells for treatment of therapy‐resistant graft‐versus‐host disease. Transplantation 81, 1390–1397. - PubMed
-
- Giordano A, Galderisi U, Marino IR (2007) From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 211, 27–35. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

