The α5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice
- PMID: 23164049
- PMCID: PMC3901849
- DOI: 10.1111/acer.12009
The α5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice
Abstract
Background: Alcohol use disorders (AUDs) are a major public health problem, and the few treatment options available to those seeking treatment offer only modest success rates. There remains a need to identify novel targets for the treatment of AUDs. The neuronal nicotinic acetylcholine receptors (nAChRs) represent a potential therapeutic target in the brain, as recent human genetic studies have implicated gene variants in the α5 nAChR subunit as high risk factors for developing alcohol dependence.
Methods: Here, we evaluate the role of the α5* nAChR for ethanol (EtOH)-mediated behaviors using male α5+/+ and α5-/- transgenic mice. We characterized the effect of hypnotic doses of EtOH and investigated drinking behavior using an adapted drinking-in-the-dark (DID) paradigm that has been shown to induce high EtOH consumption in mice.
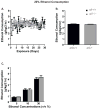
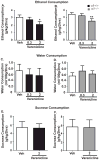
Results: We found the α5 subunit to be important in mediating the sedative effects of EtOH. The α5-/- mice showed slower recovery from EtOH-induced sleep, as measured by loss of righting reflex. Additionally, the α5-/- mice showed enhanced impairment to EtOH-induced ataxia. We found the initial sensitivity to EtOH and EtOH metabolism to be similar in both α5+/+ and α5-/- mice. Hence, the enhanced sedation is likely due to a difference in the acute tolerance of EtOH in α5-/- mice. However, the α5 subunit did not play a role in EtOH consumption for EtOH concentrations ranging from 5 to 30% using the DID paradigm. Additionally, varenicline was effective in reducing EtOH intake in α5-/- mice.
Conclusions: Together, our data suggest that the α5 nAChR subunit is important for the sedative effects of EtOH but does not play a role in EtOH consumption in male mice. Varenicline can be a treatment option even when there is loss of function of the α5 nAChR subunit.
Copyright © 2012 by the Research Society on Alcoholism.
Figures
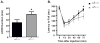

Similar articles
-
Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice.Neuropharmacology. 2018 Aug;138:341-348. doi: 10.1016/j.neuropharm.2018.06.031. Epub 2018 Jun 23. Neuropharmacology. 2018. PMID: 29944862 Free PMC article.
-
Nicotine Enhances the Hypnotic and Hypothermic Effects of Alcohol in the Mouse.Alcohol Clin Exp Res. 2016 Jan;40(1):62-72. doi: 10.1111/acer.12918. Alcohol Clin Exp Res. 2016. PMID: 26727524 Free PMC article.
-
No evidence of a role of the β4 subunit of the nicotinic acetylcholine receptor in alcohol-related behaviors.BMC Res Notes. 2017 Apr 5;10(1):151. doi: 10.1186/s13104-017-2470-7. BMC Res Notes. 2017. PMID: 28381286 Free PMC article.
-
The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice.Prog Neurobiol. 2002 Dec;68(5):341-60. doi: 10.1016/s0301-0082(02)00106-5. Prog Neurobiol. 2002. PMID: 12531234 Review.
-
P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders.Front Neurosci. 2014 Jun 24;8:176. doi: 10.3389/fnins.2014.00176. eCollection 2014. Front Neurosci. 2014. PMID: 25009459 Free PMC article. Review.
Cited by
-
The α5 subunit regulates the expression and function of α4*-containing neuronal nicotinic acetylcholine receptors in the ventral-tegmental area.PLoS One. 2013 Jul 15;8(7):e68300. doi: 10.1371/journal.pone.0068300. Print 2013. PLoS One. 2013. PMID: 23869214 Free PMC article.
-
Linking the CHRNA5 SNP to drug abuse liability: From circuitry to cellular mechanisms.Neuropharmacology. 2021 Mar 15;186:108480. doi: 10.1016/j.neuropharm.2021.108480. Epub 2021 Feb 1. Neuropharmacology. 2021. PMID: 33539855 Free PMC article. Review.
-
UFR2709, a Nicotinic Acetylcholine Receptor Antagonist, Decreases Ethanol Intake in Alcohol-Preferring Rats.Front Pharmacol. 2019 Dec 3;10:1429. doi: 10.3389/fphar.2019.01429. eCollection 2019. Front Pharmacol. 2019. PMID: 31849674 Free PMC article.
-
Profound alteration in reward processing due to a human polymorphism in CHRNA5: a role in alcohol dependence and feeding behavior.Neuropsychopharmacology. 2019 Oct;44(11):1906-1916. doi: 10.1038/s41386-019-0462-0. Epub 2019 Jul 9. Neuropsychopharmacology. 2019. PMID: 31288250 Free PMC article.
-
A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption.Front Behav Neurosci. 2017 Feb 22;11:29. doi: 10.3389/fnbeh.2017.00029. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28275340 Free PMC article.
References
-
- Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol. 1999;55:39–49. - PubMed
-
- Al-Rejaie S, Dar MS. Antagonism of ethanol ataxia by intracerebellar nicotine: possible modulation by mouse cerebellar nitric oxide and cGMP. Brain Res Bull. 2006;69:187–196. - PubMed
-
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. - PubMed
-
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

