Glucose attenuates impairments in memory and CREB activation produced by an α4β2 but not an α7 nicotinic receptor antagonist
- PMID: 23164619
- PMCID: PMC3562370
- DOI: 10.1016/j.neuropharm.2012.11.008
Glucose attenuates impairments in memory and CREB activation produced by an α4β2 but not an α7 nicotinic receptor antagonist
Abstract
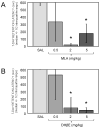
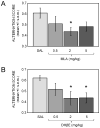
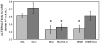
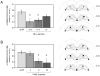
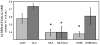
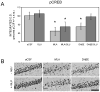
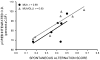
Glucose improves memory for a variety of tasks when administered to rats and mice near the time of training. Prior work indicates glucose may enhance memory by increasing the synthesis and release of the neurotransmitter acetylcholine in the brain. To investigate if specific acetylcholine receptor subtypes may mediate some of the memory-enhancing actions of glucose, we examined the effects of subtype-specific nicotinic acetylcholine receptor antagonists on memory in Fischer-344 rats and also examined the ability of glucose to reverse drug-induced impairments. Pre-training peripheral injections of methyllycaconitine (MLA) or dihydro-beta-erythroidine (DHβE), which are specific α7 and α4β2 nicotinic receptor antagonists, respectively, dose-dependently impaired retention latencies in an inhibitory avoidance task when tested 7-days but not 1 h after training. Immediate post-training glucose injections attenuated the impairments, but were more effective in attenuating the DHβE-induced impairments. Likewise, peripheral or direct intrahippocampal injections of MLA or DHβE dose-dependently impaired spatial working memory scores on a spontaneous alternation task. Concurrent administration of glucose reversed DHβE- but not MLA-induced impairments. CREB phosphorylation downstream of cholinergic signaling was assessed 30 min after spontaneous alternation testing and intrahippocampal drug infusions. Both MLA and DHβE impaired hippocampal CREB phosphorylation; glucose reversed DHβE- but not MLA-induced deficits. The effectiveness of glucose in reversing DHβE- but not MLA-induced impairments in behavioral performance and CREB phosphorylation suggests that activation of α7 receptors may play an important role in memory enhancement by glucose.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Nicotinic mechanisms of memory: effects of acute local DHbetaE and MLA infusions in the basolateral amygdala.Brain Res Cogn Brain Res. 2003 Mar;16(1):51-7. doi: 10.1016/s0926-6410(02)00209-4. Brain Res Cogn Brain Res. 2003. PMID: 12589888
-
Methyllycaconitine (MLA) blocks the nicotine evoked anxiogenic effect and 5-HT release in the dorsal hippocampus: possible role of alpha7 receptors.Neuropharmacology. 2003 Mar;44(3):367-73. doi: 10.1016/s0028-3908(02)00391-x. Neuropharmacology. 2003. PMID: 12604094
-
Ventral hippocampal alpha7 and alpha4beta2 nicotinic receptor blockade and clozapine effects on memory in female rats.Psychopharmacology (Berl). 2006 Nov;188(4):597-604. doi: 10.1007/s00213-006-0416-1. Epub 2006 May 20. Psychopharmacology (Berl). 2006. PMID: 16715255 Free PMC article.
-
Nicotinic Receptor Antagonists in Rats.In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 3. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 3. PMID: 21204359 Free Books & Documents. Review.
-
Nicotinic-antipsychotic drug interactions and cognitive function.EXS. 2006;98:185-205. doi: 10.1007/978-3-7643-7772-4_10. EXS. 2006. PMID: 17019889 Review.
Cited by
-
Forgetfulness during aging: an integrated biology.Neurobiol Learn Mem. 2014 Jul;112:130-8. doi: 10.1016/j.nlm.2014.03.005. Epub 2014 Mar 24. Neurobiol Learn Mem. 2014. PMID: 24674745 Free PMC article. Review.
-
Epinephrine May Contribute to the Persistence of Traumatic Memories in a Post-traumatic Stress Disorder Animal Model.Front Mol Neurosci. 2020 Oct 26;13:588802. doi: 10.3389/fnmol.2020.588802. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33192300 Free PMC article.
-
Acute Low Alcohol Disrupts Hippocampus-Striatum Neural Correlate of Learning Strategy by Inhibition of PKA/CREB Pathway in Rats.Front Pharmacol. 2018 Dec 6;9:1439. doi: 10.3389/fphar.2018.01439. eCollection 2018. Front Pharmacol. 2018. PMID: 30574089 Free PMC article.
-
Attenuation in rats of impairments of memory by scopolamine, a muscarinic receptor antagonist, by mecamylamine, a nicotinic receptor antagonist.Psychopharmacology (Berl). 2016 Mar;233(5):925-32. doi: 10.1007/s00213-015-4174-9. Epub 2015 Dec 10. Psychopharmacology (Berl). 2016. PMID: 26660295 Free PMC article.
-
Epinephrine and glucose modulate training-related CREB phosphorylation in old rats: relationships to age-related memory impairments.Exp Gerontol. 2013 Feb;48(2):115-27. doi: 10.1016/j.exger.2012.11.010. Epub 2012 Nov 30. Exp Gerontol. 2013. PMID: 23201424 Free PMC article.
References
-
- Addy NA, Nakijama A, Levin ED. Nicotinic mechanisms of memory: effects of acute local DHbetaE and MLA infusions in the basolateral amygdala. Brain Res Cogn Brain Res. 2003;16:51–57. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. - PubMed
-
- Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. - PubMed
-
- Bettany JH, Levin ED. Ventral hippocampal alpha 7 nicotinic receptor blockade and chronic nicotine effects on memory performance in the radial-arm maze. Pharmacol Biochem Behav. 2001;70:467–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

