On scaffold hopping: challenges in the discovery of sulfated small molecules as mimetics of glycosaminoglycans
- PMID: 23164711
- PMCID: PMC3525718
- DOI: 10.1016/j.bmcl.2012.10.079
On scaffold hopping: challenges in the discovery of sulfated small molecules as mimetics of glycosaminoglycans
Abstract
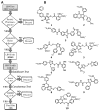
The design of sulfated, small, nonsaccharide molecules as modulators of proteins is still in its infancy as standard drug discovery tools such as library of diverse sulfated molecules and in silico docking and scoring protocol have not been firmly established. Databases, such as ZINC, contain too few sulfate-containing nonsaccharide molecules, which severely limits the identification of new hits. Lack of a generally applicable protocol for scaffold hopping limits the development of sulfated small molecules as synthetic mimetics of the highly sulfated glycosaminoglycans. We explored a sequential ligand-based (LBVS) and structure-based virtual screening (SBVS) approach starting from our initial discovery of monosulfated benzofurans to discover alternative scaffolds as allosteric modulators of thrombin, a key coagulation enzyme. Screening the ZINC database containing nearly 1 million nonsulfated small molecules using a pharmacophore developed from the parent sulfated benzofurans followed by a genetic algorithm-based dual-filter docking and scoring screening identified a group of 10 promising hits, of which three top-scoring hits were synthesized. Each was found to selectively inhibit human alpha-thrombin suggesting the possibility of this approach for scaffold hopping. Michaelis-Menten kinetics showed allosteric inhibition mechanism for the best molecule and human plasma studies confirmed good anticoagulation potential as expected. Our simple sequential LBVS and SBVS approach is likely to be useful as a general strategy for identification of sulfated small molecules hits as modulators of glycosaminoglycan-protein interactions.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




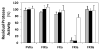

Similar articles
-
Plasmin regulation through allosteric, sulfated, small molecules.Molecules. 2015 Jan 5;20(1):608-24. doi: 10.3390/molecules20010608. Molecules. 2015. PMID: 25569517 Free PMC article.
-
A molecular dynamics-based algorithm for evaluating the glycosaminoglycan mimicking potential of synthetic, homogenous, sulfated small molecules.PLoS One. 2017 Feb 9;12(2):e0171619. doi: 10.1371/journal.pone.0171619. eCollection 2017. PLoS One. 2017. PMID: 28182755 Free PMC article.
-
Discovery of Sulfated Small Molecule Inhibitors of Matrix Metalloproteinase-8.Biomolecules. 2020 Aug 9;10(8):1166. doi: 10.3390/biom10081166. Biomolecules. 2020. PMID: 32784891 Free PMC article.
-
Designing Smaller, Synthetic, Functional Mimetics of Sulfated Glycosaminoglycans as Allosteric Modulators of Coagulation Factors.J Med Chem. 2023 Apr 13;66(7):4503-4531. doi: 10.1021/acs.jmedchem.3c00132. Epub 2023 Mar 31. J Med Chem. 2023. PMID: 37001055 Free PMC article. Review.
-
Sulfonated Penta-galloyl Glucose (SPGG): The Pharmacological Effects of Promiscuous Glycosaminoglycan Small Molecule Mimetic.Mini Rev Med Chem. 2025;25(5):365-373. doi: 10.2174/0113895575332248241030033106. Mini Rev Med Chem. 2025. PMID: 39528449 Free PMC article. Review.
Cited by
-
Supramolecular Platform Stabilizing Growth Factors.Biomacromolecules. 2018 Jul 9;19(7):2610-2617. doi: 10.1021/acs.biomac.8b00219. Epub 2018 May 2. Biomacromolecules. 2018. PMID: 29677449 Free PMC article.
-
Recent advances in employing molecular modelling to determine the specificity of glycan-binding proteins.Curr Opin Struct Biol. 2014 Oct;28:47-55. doi: 10.1016/j.sbi.2014.07.001. Epub 2014 Aug 7. Curr Opin Struct Biol. 2014. PMID: 25108191 Free PMC article. Review.
-
Asymmetric (3 + 3) and (4 + 2) Annulation Reactions of 2,3-Dioxopyrrolidines with 3-Alkylidene Oxindoles to Construct Diverse Chiral Heterocyclic Frameworks.J Org Chem. 2024 Jun 21;89(12):8970-8984. doi: 10.1021/acs.joc.4c00933. Epub 2024 Jun 8. J Org Chem. 2024. PMID: 38850251 Free PMC article.
-
Allosteric inhibition of human factor XIa: discovery of monosulfated benzofurans as a class of promising inhibitors.J Med Chem. 2014 Apr 24;57(8):3559-69. doi: 10.1021/jm5002698. Epub 2014 Apr 7. J Med Chem. 2014. PMID: 24666186 Free PMC article.
-
Rapid synthetic approaches to libraries of diversified 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones and 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones.Mol Divers. 2022 Apr;26(2):1115-1128. doi: 10.1007/s11030-021-10234-2. Epub 2021 Jun 4. Mol Divers. 2022. PMID: 34086156 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

