Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration
- PMID: 23164734
- PMCID: PMC3594364
- DOI: 10.1016/j.heares.2012.11.009
Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration
Abstract
The organ of Corti in the mammalian inner ear is comprised of mechanosensory hair cells (HCs) and nonsensory supporting cells (SCs), both of which are believed to be terminally post-mitotic beyond late embryonic ages. Consequently, regeneration of HCs and SCs does not occur naturally in the adult mammalian cochlea, though recent evidence suggests that these cells may not be completely or irreversibly quiescent at earlier postnatal ages. Furthermore, regenerative processes can be induced by genetic and pharmacological manipulations, but, more and more reports suggest that regenerative potential declines as the organ of Corti continues to age. In numerous mammalian systems, such effects of aging on regenerative potential are well established. However, in the cochlea, the problem of regeneration has not been traditionally viewed as one of aging. This is an important consideration as current models are unable to elicit widespread regeneration or full recovery of function at adult ages yet regenerative therapies will need to be developed specifically for adult populations. Still, the advent of gene targeting and other genetic manipulations has established mice as critically important models for the study of cochlear development and HC regeneration and suggests that auditory HC regeneration in adult mammals may indeed be possible. Thus, this review will focus on the pursuit of regeneration in the postnatal and adult mouse cochlea and highlight processes that occur during postnatal development, maturation, and aging that could contribute to an age-related decline in regenerative potential. Second, we will draw upon the wealth of knowledge pertaining to age related senescence in tissues outside of the ear to synthesize new insights and potentially guide future research aimed at promoting HC regeneration in the adult cochlea.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
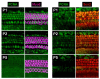

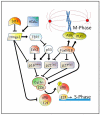
Similar articles
-
Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti.Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):4084-8. doi: 10.1073/pnas.96.7.4084. Proc Natl Acad Sci U S A. 1999. PMID: 10097167 Free PMC article.
-
Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing.J Neurosci. 2014 Nov 19;34(47):15751-63. doi: 10.1523/JNEUROSCI.3200-14.2014. J Neurosci. 2014. PMID: 25411503 Free PMC article.
-
Transcriptomic Analysis of Mouse Cochlear Supporting Cell Maturation Reveals Large-Scale Changes in Notch Responsiveness Prior to the Onset of Hearing.PLoS One. 2016 Dec 5;11(12):e0167286. doi: 10.1371/journal.pone.0167286. eCollection 2016. PLoS One. 2016. PMID: 27918591 Free PMC article.
-
Manipulating cell fate in the cochlea: a feasible therapy for hearing loss.Trends Neurosci. 2015 Mar;38(3):139-44. doi: 10.1016/j.tins.2014.12.004. Epub 2015 Jan 12. Trends Neurosci. 2015. PMID: 25593106 Free PMC article. Review.
-
Hair cell fate decisions in cochlear development and regeneration.Hear Res. 2010 Jul;266(1-2):18-25. doi: 10.1016/j.heares.2010.04.012. Epub 2010 May 5. Hear Res. 2010. PMID: 20438823 Free PMC article. Review.
Cited by
-
Hypoxia Induces a Metabolic Shift and Enhances the Stemness and Expansion of Cochlear Spiral Ganglion Stem/Progenitor Cells.Biomed Res Int. 2015;2015:359537. doi: 10.1155/2015/359537. Epub 2015 Jul 5. Biomed Res Int. 2015. PMID: 26236724 Free PMC article.
-
Advances in genetic hearing loss: CIB2 gene.Eur Arch Otorhinolaryngol. 2017 Apr;274(4):1791-1795. doi: 10.1007/s00405-016-4330-9. Epub 2016 Oct 22. Eur Arch Otorhinolaryngol. 2017. PMID: 27771768 Free PMC article. Review.
-
Postnatal Development of the Subcellular Structures and Purinergic Signaling of Deiters' Cells along the Tonotopic Axis of the Cochlea.Cells. 2019 Oct 17;8(10):1266. doi: 10.3390/cells8101266. Cells. 2019. PMID: 31627326 Free PMC article.
-
Jag1 represses Notch activation in lateral supporting cells and inhibits an outer hair cell fate in the medial cochlea.Development. 2024 Nov 1;151(21):dev202949. doi: 10.1242/dev.202949. Epub 2024 Nov 5. Development. 2024. PMID: 39373109 Free PMC article.
-
Sensory hair cell development and regeneration: similarities and differences.Development. 2015 May 1;142(9):1561-71. doi: 10.1242/dev.114926. Development. 2015. PMID: 25922522 Free PMC article. Review.
References
-
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochemical and biophysical research communications. 2005;335:485–90. - PubMed
-
- Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, Yamamoto E, Tarasawa I, Sonoda T, Mori M, Imai K, Shinomura Y, Tokino T. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer science. 2007;98:88–95. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

