Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing
- PMID: 23164936
- PMCID: PMC3594010
- DOI: 10.1038/mt.2012.234
Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing
Abstract
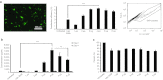
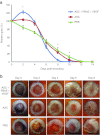
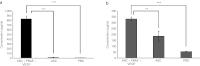
Angiogenesis is essential to wound repair, and vascular endothelial growth factor (VEGF) is a potent factor to stimulate angiogenesis. Here, we examine the potential of VEGF-overexpressing adipose-derived stromal cells (ASCs) for accelerating wound healing using nonviral, biodegradable polymeric vectors. Mouse ASCs were transfected with DNA plasmid encoding VEGF or green fluorescent protein (GFP) using biodegradable poly (β-amino) esters (PBAE). Cells transfected using Lipofectamine 2000, a commercially available transfection reagent, were included as controls. ASCs transfected using PBAEs showed enhanced transfection efficiency and 12-15-fold higher VEGF production compared with cells transfected using Lipofectamine 2000 (*P < 0.05). When transplanted into a mouse wild-type excisional wound model, VEGF-overexpressing ASCs led to significantly accelerated wound healing, with full wound closure observed at 8 days compared to 10-12 days in groups treated with ASCs alone or saline control (*P < 0.05). Histology and polarized microscopy showed increased collagen deposition and more mature collagen fibers in the dermis of wound beds treated using PBAE/VEGF-modified ASCs than ASCs alone. Our results demonstrate the efficacy of using nonviral-engineered ASCs to accelerate wound healing, which may provide an alternative therapy for treating many diseases in which wound healing is impaired.
Figures





Similar articles
-
Paracrine release from nonviral engineered adipose-derived stem cells promotes endothelial cell survival and migration in vitro.Stem Cells Dev. 2013 Feb 1;22(3):483-91. doi: 10.1089/scd.2012.0201. Epub 2012 Oct 5. Stem Cells Dev. 2013. PMID: 22889246 Free PMC article.
-
Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice.Stem Cell Res Ther. 2020 Mar 17;11(1):120. doi: 10.1186/s13287-020-01621-x. Stem Cell Res Ther. 2020. PMID: 32183899 Free PMC article.
-
Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis.Cell Transplant. 2011;20(2):205-16. doi: 10.3727/096368910X520065. Epub 2010 Aug 18. Cell Transplant. 2011. PMID: 20719083
-
Adipose-derived stem cells for wound healing.J Cell Physiol. 2019 Jun;234(6):7903-7914. doi: 10.1002/jcp.27922. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515810 Review.
-
The role of vascular endothelial growth factor in wound healing.J Surg Res. 2009 May 15;153(2):347-58. doi: 10.1016/j.jss.2008.04.023. Epub 2008 May 12. J Surg Res. 2009. PMID: 19027922 Free PMC article. Review.
Cited by
-
EGF and curcumin co-encapsulated nanoparticle/hydrogel system as potent skin regeneration agent.Int J Nanomedicine. 2016 Aug 17;11:3993-4009. doi: 10.2147/IJN.S104350. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27574428 Free PMC article.
-
The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats.Cell Transplant. 2019 Jan;28(1):105-115. doi: 10.1177/0963689718807410. Epub 2018 Oct 23. Cell Transplant. 2019. PMID: 30350716 Free PMC article.
-
Nanoparticle-mediated transcriptional modification enhances neuronal differentiation of human neural stem cells following transplantation in rat brain.Biomaterials. 2016 Apr;84:157-166. doi: 10.1016/j.biomaterials.2016.01.037. Epub 2016 Jan 20. Biomaterials. 2016. PMID: 26828681 Free PMC article.
-
PHD-2 Suppression in Mesenchymal Stromal Cells Enhances Wound Healing.Plast Reconstr Surg. 2018 Jan;141(1):55e-67e. doi: 10.1097/PRS.0000000000003959. Plast Reconstr Surg. 2018. PMID: 29280872 Free PMC article.
-
The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches.Annu Rev Cell Dev Biol. 2016 Oct 6;32:609-631. doi: 10.1146/annurev-cellbio-111315-125426. Epub 2016 May 4. Annu Rev Cell Dev Biol. 2016. PMID: 27146311 Free PMC article. Review.
References
-
- Gurtner GC, Werner S, Barrandon Y., and, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

