Particle tracking analysis for the intracellular trafficking of nanoparticles modified with African swine fever virus protein p54-derived peptide
- PMID: 23164937
- PMCID: PMC3594009
- DOI: 10.1038/mt.2012.235
Particle tracking analysis for the intracellular trafficking of nanoparticles modified with African swine fever virus protein p54-derived peptide
Abstract
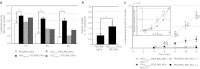
Previous studies showed that the cytoplasmic transport of nanoparticles to the nucleus is driven by a vesicular sorting system. Artificial approaches for targeting a microtubule-associating motor complex is also a challenge. We describe herein the development of a liposomal nanoparticle, the surface of which is modified with stearylated octa-arginine (STR-R8), and a dynein light chain (LC8)-associated peptide derived from an African swine fever virus protein p54 (p54(149-161)) with polyethyleneglycol (PEG) as a spacer (p54(149-161)-PEG/R8-liposomal nanoparticles (LNPs)). The p54(149-161)-PEG/R8-LNPs preferentially gain access to the nucleus, resulting in a one- to two-order of magnitude higher transfection activity in comparison with p54(149-161)-free nanoparticles (PEG/R8-LNPs). Further studies of particle tracking in HeLa cells stably expressing green fluorescent protein (GFP)-tagged tubulin (GFP/Tub-HeLa) indicate that p54(149-161) stimulated the transport of nanoparticles along fibrous tubulin structures. Moreover, a part of the p54(149-161)-PEG/R8-LNPs appeared to undergo quasi-straight transport without sharing the tracks corresponding to PKH67, the plasma membrane of which had been prestained with a marker just before transfection, while corresponding movement was never observed in the case of PEG/R8-LNPs. These findings suggest that a portion of the p54(149-161)-modified nanoparticles can use microtubule-dependent transport without the need for an assist by a vesicular sorting system.
Figures


Similar articles
-
Particle tracking of intracellular trafficking of octaarginine-modified liposomes: a comparative study with adenovirus.Mol Ther. 2010 May;18(5):955-64. doi: 10.1038/mt.2010.33. Epub 2010 Mar 9. Mol Ther. 2010. PMID: 20216528 Free PMC article.
-
Stearylated INF7 peptide enhances endosomal escape and gene expression of PEGylated nanoparticles both in vitro and in vivo.J Pharm Sci. 2012 Feb;101(2):879-82. doi: 10.1002/jps.22807. Epub 2011 Nov 15. J Pharm Sci. 2012. PMID: 22086751
-
African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein.J Virol. 2001 Oct;75(20):9819-27. doi: 10.1128/JVI.75.20.9819-9827.2001. J Virol. 2001. PMID: 11559815 Free PMC article.
-
Development and characterization of monoclonal antibodies against the N-terminal domain of African swine fever virus structural protein, p54.Int J Biol Macromol. 2021 Jun 1;180:203-211. doi: 10.1016/j.ijbiomac.2021.03.059. Epub 2021 Mar 16. Int J Biol Macromol. 2021. PMID: 33737177
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
In vivo characterization of dynein-driven nanovectors using Drosophila oocytes.PLoS One. 2013 Dec 12;8(12):e82908. doi: 10.1371/journal.pone.0082908. eCollection 2013. PLoS One. 2013. PMID: 24349395 Free PMC article.
-
Synthetic Approaches for Nucleic Acid Delivery: Choosing the Right Carriers.Life (Basel). 2019 Jul 9;9(3):59. doi: 10.3390/life9030059. Life (Basel). 2019. PMID: 31324016 Free PMC article. Review.
-
Nanoparticles engineered to bind cellular motors for efficient delivery.J Nanobiotechnology. 2018 Mar 30;16(1):33. doi: 10.1186/s12951-018-0354-1. J Nanobiotechnology. 2018. PMID: 29602307 Free PMC article.
-
Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19-9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer.Theranostics. 2016 Jun 18;6(10):1573-87. doi: 10.7150/thno.15164. eCollection 2016. Theranostics. 2016. PMID: 27446491 Free PMC article.
-
Imaging, Tracking and Computational Analyses of Virus Entry and Egress with the Cytoskeleton.Viruses. 2018 Mar 31;10(4):166. doi: 10.3390/v10040166. Viruses. 2018. PMID: 29614729 Free PMC article. Review.
References
-
- Kamiya H, Akita H., and, Harashima H. Pharmacokinetic and pharmacodynamic considerations in gene therapy. Drug Discov Today. 2003;8:990–996. - PubMed
-
- Mastrobattista E, van der Aa MA, Hennink WE., and, Crommelin DJ. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 2006;5:115–121. - PubMed
-
- Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N., and, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275:1625–1629. - PubMed
-
- Dauty E., and, Verkman AS. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: a new barrier for non-viral gene delivery. J Biol Chem. 2005;280:7823–7828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

