Polyethylenimine-modified pluronics (PCMs) improve morpholino oligomer delivery in cell culture and dystrophic mdx mice
- PMID: 23164938
- PMCID: PMC3538317
- DOI: 10.1038/mt.2012.236
Polyethylenimine-modified pluronics (PCMs) improve morpholino oligomer delivery in cell culture and dystrophic mdx mice
Abstract
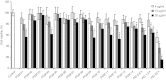
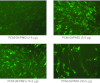
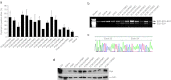
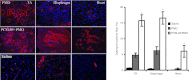
We investigated a series of small-sized polyethylenimine (PEI, 0.8/1.2 k)-conjugated pluronic copolymers (PCMs) for their potential to enhance delivery of an antisense phosphorodiamidate morpholino oligomer (PMO) in vitro and in dystrophic mdx mice. PCM polymers containing pluronics of molecular weight (Mw) ranging 2-6 k, with hydrophilic-lipophilic balance (HLB) 7-23, significantly enhanced PMO-induced exon-skipping in a green fluorescent protein (GFP) reporter-based myoblast culture system. Application of optimized formulations of PCMs with PMO targeted to dystrophin exon 23 demonstrated a significant increase in exon-skipping efficiency in dystrophic mdx mice. Consistent with our observations in vitro, optimization of molecular size and the HLB of pluronics are important factors for PCMs to achieve enhanced PMO delivery in vivo. Observed cytotoxicity of the PCMs was lower than Endo-porter and PEI 25 k. Tissue toxicity of PCMs in muscle was not clearly detected with the concentrations used, indicating the potential of the PCMs as effective and safe PMO carriers for treating diseases such as muscular dystrophy.
Figures



References
-
- Hoffman EP, Brown RH., Jr, and, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

