Pro- and anti-apoptotic effects of p53 in cisplatin-treated human testicular cancer are cell context-dependent
- PMID: 23165211
- PMCID: PMC3562300
- DOI: 10.4161/cc.22803
Pro- and anti-apoptotic effects of p53 in cisplatin-treated human testicular cancer are cell context-dependent
Abstract
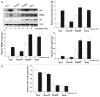
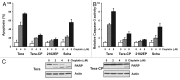
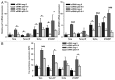
In murine testicular cancer (TC) cells wild-type p53 contributes to sensitivity to DNA-damaging drugs in a dose-dependent way. In human TC, however, the role of wild-type p53 functionality in chemotherapeutic response remains elusive. We analyzed functionality of wild-type p53 in cisplatin sensitivity in the human TC setting using a p53 short interfering (si)RNA approach. The cisplatin-sensitive TC cell line (Tera), the subline with acquired cisplatin resistance (Tera-CP) and a panel of intrinsically resistant TC cell lines (Scha and 2102EP), all expressing wild-type p53, were used. p53 and p53 transcriptional targets MDM2 and p21 (Waf1/Cip1) (p21) were expressed in a p53 transactivation-dependent way in all TC cell lines. Following cisplatin exposure, expression levels of p53 increased, with a subsequent increase in MDM2 and p21 mRNA and protein levels and Fas cell membrane levels. Downregulation of p53 with siRNA lowered cisplatin-induced apoptosis in Tera and Tera-CP, which was associated with a diminished Fas membrane expression. In contrast, p53 suppression augmented cisplatin-induced apoptosis in Scha and 2102EP and concomitantly strongly suppressed MDM2 and p21 mRNA and protein expression. Our results indicate that p53 is involved in transactivation of pro- and anti-apoptotic genes in untreated and cisplatin-treated TC cells, but subtle differences are present between TC cell lines. The opposite role of p53 in cisplatin-induced apoptosis among TC cell lines demonstrates the importance of the cellular context for the p53 transactivation phenotype in TC cells.
Figures



Similar articles
-
Disruption of the MDM2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the Fas/FasL pathway.Cell Death Dis. 2011 Apr 21;2(4):e148. doi: 10.1038/cddis.2011.33. Cell Death Dis. 2011. PMID: 21509038 Free PMC article.
-
Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against Cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and Caspase-9/-3 activation.Am J Physiol Cell Physiol. 2016 Jun 1;310(11):C857-73. doi: 10.1152/ajpcell.00256.2015. Epub 2016 Mar 16. Am J Physiol Cell Physiol. 2016. PMID: 26984736 Free PMC article.
-
Low p21Waf1/Cip1 protein level sensitizes testicular germ cell tumor cells to Fas-mediated apoptosis.Oncogene. 2004 Jun 17;23(28):4862-72. doi: 10.1038/sj.onc.1207617. Oncogene. 2004. PMID: 15122333
-
Testicular germ cell tumours: the paradigm of chemo-sensitive solid tumours.Int J Biochem Cell Biol. 2005 Dec;37(12):2437-56. doi: 10.1016/j.biocel.2005.06.014. Epub 2005 Aug 11. Int J Biochem Cell Biol. 2005. PMID: 16099193 Review.
-
Unravelling mechanisms of cisplatin sensitivity and resistance in testicular cancer.Expert Rev Mol Med. 2013 Sep 30;15:e12. doi: 10.1017/erm.2013.13. Expert Rev Mol Med. 2013. PMID: 24074238 Review.
Cited by
-
Comparison of cisplatin-induced anti-tumor response in CT26 syngeneic tumors of three BALB/c substrains.Lab Anim Res. 2021 Dec 8;37(1):33. doi: 10.1186/s42826-021-00110-3. Lab Anim Res. 2021. PMID: 34876239 Free PMC article.
-
Bromate-induced Changes in p21 DNA Methylation and Histone Acetylation in Renal Cells.Toxicol Sci. 2019 Apr 1;168(2):460-473. doi: 10.1093/toxsci/kfz016. Toxicol Sci. 2019. PMID: 30649504 Free PMC article.
-
Downregulation of O-GlcNAcylation enhances etoposide-induced p53-mediated apoptosis in HepG2 human liver cancer cells.FEBS Open Bio. 2025 Jul;15(7):1176-1188. doi: 10.1002/2211-5463.70028. Epub 2025 Apr 16. FEBS Open Bio. 2025. PMID: 40237201 Free PMC article.
-
Absence of REV3L promotes p53-regulated cancer cell metabolism in cisplatin-treated lung carcinoma cells.Biochem Biophys Res Commun. 2018 Jan 29;496(1):199-204. doi: 10.1016/j.bbrc.2018.01.026. Epub 2018 Jan 4. Biochem Biophys Res Commun. 2018. PMID: 29307819 Free PMC article.
-
Therapeutic Challenges for Cisplatin-Resistant Ovarian Germ Cell Tumors.Cancers (Basel). 2019 Oct 17;11(10):1584. doi: 10.3390/cancers11101584. Cancers (Basel). 2019. PMID: 31627378 Free PMC article. Review.
References
-
- Pottern A. Testicular and penile cancer. Ernstoff Heaney Peschel 1998.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
