The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer
- PMID: 23165325
- PMCID: PMC3625515
- DOI: 10.1002/ijc.27950
The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer
Abstract
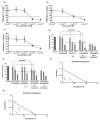
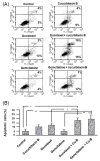
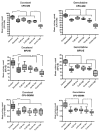
Despite recent advances in therapy, breast cancer remains the second most common cause of death from malignancy in women. Chemotherapy plays a major role in breast cancer management, and combining chemotherapeutic agents with nonchemotherapeutic agents is of considerable clinical interest. Cucurbitacins are triterpenes compounds found in plants of the Cucurbitaceae family, reported to have anticancer and anti-inflammatory activities. Previously, we have shown antiproliferative activity of cucurbitacin B (CuB) in breast cancer, and we hypothesized that combining CuB with chemotherapeutic agents can augment their antitumor effect. Here, we show that a combination of CuB with either docetaxel (DOC) or gemcitabine (GEM) synergistically inhibited the proliferation of MDA-MB-231 breast cancer cells in vitro. This antiproliferative effect was accompanied by an increase in apoptosis rates. Furthermore, in vivo treatment of human breast cancer orthotopic xenografts in immunodeficient mice with CuB at either low (0.5 mg/kg) or high (1 mg/kg) doses in combination with either DOC (20 mg/kg) or GEM (12.5mg/kg) significantly reduced tumor volume as compared with monotherapy of each drug. Importantly, no significant toxicity was noted with low-dose CuB in combination with either DOC or GEM. In conclusion, combination of CuB at a relatively low concentration with either of the chemotherapeutic agents, DOC or GEM, shows prominent antiproliferative activity against breast cancer cells without increased toxicity. This promising combination should be examined in therapeutic trials of breast cancer.
Copyright © 2012 UICC.
Conflict of interest statement
Conflict of interest: At the time of the preparation of this manuscript M Toh and KT Chan were employees of CK Life Sciences, Inc.
Figures

Similar articles
-
Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer.Br J Pharmacol. 2010 Jun;160(4):998-1007. doi: 10.1111/j.1476-5381.2010.00741.x. Br J Pharmacol. 2010. PMID: 20590594 Free PMC article.
-
Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells.Cancer Res. 2009 Jul 15;69(14):5876-84. doi: 10.1158/0008-5472.CAN-09-0536. Cancer Res. 2009. PMID: 19605406
-
Smac mimetic-derived augmentation of chemotherapeutic response in experimental pancreatic cancer.BMC Cancer. 2011 Jan 12;11:15. doi: 10.1186/1471-2407-11-15. BMC Cancer. 2011. PMID: 21226944 Free PMC article.
-
Bevacizumab in combination with a taxane for the first-line treatment of HER2-negative metastatic breast cancer.Health Technol Assess. 2011 May;15 Suppl 1:1-12. doi: 10.3310/hta15suppl1/01. Health Technol Assess. 2011. PMID: 21609648 Review.
-
Unveiling the pharmacological potential of plant triterpenoids in breast cancer management: an updated review.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5571-5596. doi: 10.1007/s00210-024-03054-2. Epub 2024 Apr 2. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38563878 Free PMC article. Review.
Cited by
-
Characterization and anti-uterine tumor effect of extract from Prunella vulgaris L.BMC Complement Med Ther. 2020 Jun 18;20(1):189. doi: 10.1186/s12906-020-02986-5. BMC Complement Med Ther. 2020. PMID: 32552673 Free PMC article.
-
Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine.Chin Med. 2019 Nov 6;14:48. doi: 10.1186/s13020-019-0270-9. eCollection 2019. Chin Med. 2019. PMID: 31719837 Free PMC article. Review.
-
Cucurbitacins as Potent Chemo-Preventive Agents: Mechanistic Insight and Recent Trends.Biomolecules. 2022 Dec 27;13(1):57. doi: 10.3390/biom13010057. Biomolecules. 2022. PMID: 36671442 Free PMC article. Review.
-
Cucurbitacin B and cisplatin induce the cell death pathways in MB49 mouse bladder cancer model.Exp Biol Med (Maywood). 2020 May;245(9):805-814. doi: 10.1177/1535370220917367. Epub 2020 Apr 6. Exp Biol Med (Maywood). 2020. PMID: 32252554 Free PMC article.
-
Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents.Front Endocrinol (Lausanne). 2021 Jan 21;11:612396. doi: 10.3389/fendo.2020.612396. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33552000 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–39. Review. - PubMed
-
- Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S. Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol. 2006;24:4963–70. - PubMed
-
- Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, Harris AL. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 1995;13:2731–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

