Intensification of antiretroviral therapy through addition of enfuvirtide in naive HIV-1-infected patients with severe immunosuppression does not improve immunological response: results of a randomized multicenter trial (ANRS 130 Apollo)
- PMID: 23165467
- PMCID: PMC3553717
- DOI: 10.1128/AAC.01662-12
Intensification of antiretroviral therapy through addition of enfuvirtide in naive HIV-1-infected patients with severe immunosuppression does not improve immunological response: results of a randomized multicenter trial (ANRS 130 Apollo)
Abstract
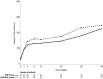
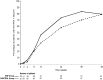
We studied whether addition of enfuvirtide (ENF) to a background combination antiretroviral therapy (cART) would improve the CD4 cell count response at week 24 in naive patients with advanced HIV disease. ANRS 130 Apollo is a randomized study, conducted in naive HIV-1-infected patients, either asymptomatic with CD4 counts of <100/mm(3) or stage B/C disease with CD4 counts of <200/mm(3). Patients received tenofovir-emtricitabine with lopinavir-ritonavir (LPV/r) or efavirenz and were randomized to receive ENF for 24 weeks (ENF arm) or not (control arm). The primary endpoint was the proportion of patients with CD4 counts of ≥ 200/mm(3) at week 24. A total of 195 patients were randomized: 73% had stage C disease, 78% were male, the mean age was 44 years, the median CD4 count was 30/mm(3), and the median HIV-1 RNA load was 5.4 log(10) copies/ml. Eighty-one percent of patients received LPV/r. One patient was lost to follow-up, and eight discontinued the study (four in each arm). The proportions of patients with CD4 counts of ≥ 200/mm(3) at week 24 were 34% and 38% in the ENF and control arms, respectively (P = 0.53). The proportions of patients with HIV-1 RNA loads of <50 copies/ml were 74% and 58% at week 24 in the ENF and control arms, respectively (P < 0.02), and the proportion reached 79% in both arms at week 48. Twenty (20%) and 12 patients (13%) in the ENF and control arms, respectively, experienced at least one AIDS event during follow-up (P = 0.17). Although inducing a more rapid virological response, addition of ENF to a standard cART does not improve the immunological outcome in naive HIV-infected patients with severe immunosuppression.
Figures

References
-
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, HIV Outpatient Study Investigators 2006. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 43:27–34 - PubMed
-
- Hirsch MS, Steigbigel RT, Staszewski S, McMahon D, Fischl MA, Hirschel B, Squires K, Dinubile NJ, Harvey CM, Chen J, Leavitt RY, Protocol 039 Study Team 2003. Long-term efficacy, safety, and tolerability of indinavir-based therapy in protease inhibitor-naive adults with advanced HIV infection. Clin. Infect. Dis. 37:1119–1124 - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, Sun E, Henry D, Rode R, Potthoff A, Leonard J. 1998. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet 35:543–549 - PubMed
-
- Egger M, May T, Chêne G, Phillips AN, Lederberger B, Dabis F, Costagliola D, D'Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA, Cohort Collaboration ART 2002. Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360:119–129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

