Human pericytes for ischemic heart repair
- PMID: 23165704
- PMCID: PMC3572307
- DOI: 10.1002/stem.1285
Human pericytes for ischemic heart repair
Abstract
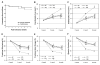
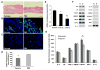
Human microvascular pericytes (CD146(+)/34(-)/45(-)/56(-)) contain multipotent precursors and repair/regenerate defective tissues, notably skeletal muscle. However, their ability to repair the ischemic heart remains unknown. We investigated the therapeutic potential of human pericytes, purified from skeletal muscle, for treating ischemic heart disease and mediating associated repair mechanisms in mice. Echocardiography revealed that pericyte transplantation attenuated left ventricular dilatation and significantly improved cardiac contractility, superior to CD56+ myogenic progenitor transplantation, in acutely infarcted mouse hearts. Pericyte treatment substantially reduced myocardial fibrosis and significantly diminished infiltration of host inflammatory cells at the infarct site. Hypoxic pericyte-conditioned medium suppressed murine fibroblast proliferation and inhibited macrophage proliferation in vitro. High expression by pericytes of immunoregulatory molecules, including interleukin-6, leukemia inhibitory factor, cyclooxygenase-2, and heme oxygenase-1, was sustained under hypoxia, except for monocyte chemotactic protein-1. Host angiogenesis was significantly increased. Pericytes supported microvascular structures in vivo and formed capillary-like networks with/without endothelial cells in three-dimensional cocultures. Under hypoxia, pericytes dramatically increased expression of vascular endothelial growth factor-A, platelet-derived growth factor-β, transforming growth factor-β1 and corresponding receptors while expression of basic fibroblast growth factor, hepatocyte growth factor, epidermal growth factor, and angiopoietin-1 was repressed. The capacity of pericytes to differentiate into and/or fuse with cardiac cells was revealed by green fluorescence protein labeling, although to a minor extent. In conclusion, intramyocardial transplantation of purified human pericytes promotes functional and structural recovery, attributable to multiple mechanisms involving paracrine effects and cellular interactions.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
J.H. received remuneration from Cook MyoSite, Inc. for consulting services and for royalties received from technology licensing during the period that the above research was performed. All other authors have no conflict of interest to disclose.
Figures





Similar articles
-
Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair.Circ Res. 2015 May 8;116(10):e81-94. doi: 10.1161/CIRCRESAHA.115.306146. Epub 2015 Mar 23. Circ Res. 2015. PMID: 25801898
-
Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132.Circ Res. 2011 Sep 30;109(8):894-906. doi: 10.1161/CIRCRESAHA.111.251546. Epub 2011 Aug 25. Circ Res. 2011. PMID: 21868695 Free PMC article.
-
Cardiac Pericytes Acquire a Fibrogenic Phenotype and Contribute to Vascular Maturation After Myocardial Infarction.Circulation. 2023 Sep 12;148(11):882-898. doi: 10.1161/CIRCULATIONAHA.123.064155. Epub 2023 Jun 23. Circulation. 2023. PMID: 37350296 Free PMC article.
-
Pericytes from human veins for treatment of myocardial ischemia.Trends Cardiovasc Med. 2013 Apr;23(3):66-70. doi: 10.1016/j.tcm.2012.09.002. Epub 2013 Jan 10. Trends Cardiovasc Med. 2013. PMID: 23313330 Free PMC article. Review.
-
Discovering cardiac pericyte biology: From physiopathological mechanisms to potential therapeutic applications in ischemic heart disease.Vascul Pharmacol. 2016 Nov;86:53-63. doi: 10.1016/j.vph.2016.05.009. Epub 2016 Jun 5. Vascul Pharmacol. 2016. PMID: 27268036 Review.
Cited by
-
Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes.Biomed Res Int. 2018 Oct 10;2018:6740408. doi: 10.1155/2018/6740408. eCollection 2018. Biomed Res Int. 2018. PMID: 30406137 Free PMC article. Review.
-
Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes.Front Cardiovasc Med. 2018 Jun 27;5:78. doi: 10.3389/fcvm.2018.00078. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 29998128 Free PMC article. Review.
-
A role for pericytes in coronary no-reflow.Nat Rev Cardiol. 2014 Jul;11(7):427-32. doi: 10.1038/nrcardio.2014.58. Epub 2014 Apr 29. Nat Rev Cardiol. 2014. PMID: 24776704 Review.
-
Matters of the heart: Cellular sex differences.J Mol Cell Cardiol. 2021 Nov;160:42-55. doi: 10.1016/j.yjmcc.2021.04.010. Epub 2021 Jun 22. J Mol Cell Cardiol. 2021. PMID: 34166708 Free PMC article. Review.
-
Leukemia Inhibitory Factor Protects Neurons from Ischemic Damage via Upregulation of Superoxide Dismutase 3.Mol Neurobiol. 2017 Jan;54(1):608-622. doi: 10.1007/s12035-015-9587-2. Epub 2016 Jan 9. Mol Neurobiol. 2017. PMID: 26746670 Free PMC article.
References
-
- Kumar V, Fausto N, Abbas A. Robbins & Cotran Pathologic Basis of Disease. 7. Philadelphia, PA: Saunders; 2004.
-
- Hansson EM, Lindsay ME, Chien KR. Regeneration Next: Toward Heart Stem Cell Therapeutics. Cell Stem Cell. 2009;5(4):364–377. - PubMed
-
- Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. - PubMed
-
- Janssens S. Stem Cells in the Treatment of Heart Disease. Annual Review of Medicine. 2010;61(1):287–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

