Tensor tympani motoneurons receive mostly excitatory synaptic inputs
- PMID: 23165747
- PMCID: PMC3896885
- DOI: 10.1002/ar.22620
Tensor tympani motoneurons receive mostly excitatory synaptic inputs
Abstract
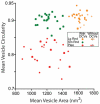
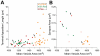
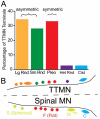
The tensor tympani is a middle ear muscle that contracts in two different situations: in response to sound or during voluntary movements. To gain insight into the inputs and neural regulation of the tensor tympani, we examined the ultrastructure of synaptic terminals on labeled tensor tympani motoneurons (TTMNs) using transmission electron microscopy. Our sample of six TTMNs received 79 synaptic terminals that formed 126 synpases. Two types of synapses are associated with round vesicles and form asymmetric junctions (excitatory morphology). One of these types has vesicles that are large and round (Lg Rnd) and the other has vesicles that are smaller and round (Sm Rnd) and also contains at least one dense core vesicle. A third synapse type has inhibitory morphology because it forms symmetric synapses with pleomorphic vesicles (Pleo). These synaptic terminals can be associated with TTMN spines. Two other types of synapse are found on TTMNs but they are uncommon. Synaptic terminals of all types form multiple synapses but those from a single terminal are always the same type. Terminals with Lg Rnd vesicles formed the most synpases per terminal (avg. 2.73). Together, the synaptic terminals with Lg Rnd and Sm Rnd vesicles account for 62% of the terminals on TTMNs, and they likely represent the pathways driving the contractions in response to sound or during voluntary movements. Having a high proportion of excitatory inputs, the TTMN innervation is like that of stapedius motoneurons but proportionately different from other types of motoneurons.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


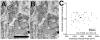


Similar articles
-
Ultrastructure of spines and associated terminals on brainstem neurons controlling auditory input.Brain Res. 2013 Jun 21;1516:1-10. doi: 10.1016/j.brainres.2013.04.020. Epub 2013 Apr 18. Brain Res. 2013. PMID: 23602963 Free PMC article.
-
Diverse synaptic terminals on rat stapedius motoneurons.J Assoc Res Otolaryngol. 2008 Sep;9(3):321-33. doi: 10.1007/s10162-008-0125-z. Epub 2008 Jun 18. J Assoc Res Otolaryngol. 2008. PMID: 18563488 Free PMC article.
-
A morphologic study of Fluorogold labeled tensor tympani motoneurons in mice.Brain Res. 2009 Jun 30;1278:59-65. doi: 10.1016/j.brainres.2009.04.035. Epub 2009 May 3. Brain Res. 2009. PMID: 19397898 Free PMC article.
-
Serotoninergic innervation of stapedial and tensor tympani motoneurons.Brain Res. 1998 Mar 16;787(1):175-8. doi: 10.1016/s0006-8993(97)01020-2. Brain Res. 1998. PMID: 9518599 Review.
-
Auditory brainstem circuits that mediate the middle ear muscle reflex.Trends Amplif. 2010 Sep;14(3):170-91. doi: 10.1177/1084713810381771. Epub 2010 Sep 23. Trends Amplif. 2010. PMID: 20870664 Free PMC article. Review.
Cited by
-
The function of the tensor tympani muscle: a comprehensive review of the literature.Anat Cell Biol. 2022 Jun 30;55(2):113-117. doi: 10.5115/acb.21.032. Epub 2022 May 19. Anat Cell Biol. 2022. PMID: 35586903 Free PMC article. Review.
-
Ultrastructure of spines and associated terminals on brainstem neurons controlling auditory input.Brain Res. 2013 Jun 21;1516:1-10. doi: 10.1016/j.brainres.2013.04.020. Epub 2013 Apr 18. Brain Res. 2013. PMID: 23602963 Free PMC article.
References
-
- Ahmari S, Buchanan J, Smith S. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Bae YC, Tatsuzo N, Ihn HJ, Choi MH, Yoshida A, Moritani M, Shiho H, Shigenaga Y. Distribution pattern of inhibitory and excitatory synapses in the dendritic tree of single masseter alpha-motoneurons in the cat. J Comp Neurol. 1999;414:454–468. - PubMed
-
- Benson TE, Berglund AM, Brown MC. Synaptic input to cochlear-nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol. 1996;365:27–41. - PubMed
-
- Berrebi AS, Spirou GA. PEP-19 immunoreactivity in the cochlear nucleus and superior olive of the cat. Neuroscience. 1998;83:535–554. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

