Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells
- PMID: 23165769
- PMCID: PMC3577540
- DOI: 10.1113/jphysiol.2012.238014
Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells
Abstract
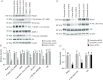
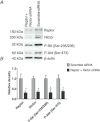
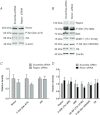
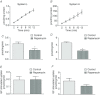
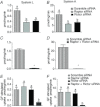
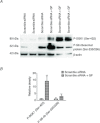
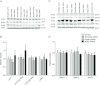
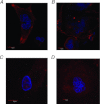
Abnormal fetal growth increases the risk for perinatal complications and predisposes for the development of obesity, diabetes and cardiovascular disease later in life. Emerging evidence suggests that changes in placental amino acid transport directly contribute to altered fetal growth. However, the molecular mechanisms regulating placental amino acid transport are largely unknown. Here we combined small interfering (si) RNA-mediated silencing approaches with protein expression/localization and functional studies in cultured primary human trophoblast cells to test the hypothesis that mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) regulate amino acid transporters by post-translational mechanisms. Silencing raptor (inhibits mTORC1) or rictor (inhibits mTORC2) markedly decreased basal System A and System L amino acid transport activity but had no effect on growth factor-stimulated amino acid uptake. Simultaneous inhibition of mTORC1 and 2 completely inhibited both basal and growth factor-stimulated amino acid transport activity. In contrast, mTOR inhibition had no effect on serotonin transport. mTORC1 or mTORC2 silencing markedly decreased the plasma membrane expression of specific System A (SNAT2, SLC38A2) and System L (LAT1, SLC7A5) transporter isoforms without affecting global protein expression. In conclusion, mTORC1 and mTORC2 regulate human trophoblast amino acid transporters by modulating the cell surface abundance of specific transporter isoforms. This is the first report showing regulation of amino acid transport by mTORC2. Because placental mTOR activity and amino acid transport are decreased in human intrauterine growth restriction our data are consistent with the possibility that dysregulation of placental mTOR plays an important role in the development of abnormal fetal growth.
Figures

Similar articles
-
Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2.Clin Sci (Lond). 2016 Apr 1;130(7):499-512. doi: 10.1042/CS20150554. Epub 2015 Nov 25. Clin Sci (Lond). 2016. PMID: 26608079 Free PMC article.
-
Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC.Sci Rep. 2016 Aug 26;6:31705. doi: 10.1038/srep31705. Sci Rep. 2016. PMID: 27562465 Free PMC article.
-
Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR.Clin Sci (Lond). 2015 Dec;129(12):1131-41. doi: 10.1042/CS20150511. Epub 2015 Sep 15. Clin Sci (Lond). 2015. PMID: 26374858 Free PMC article.
-
Mechanistic Target of Rapamycin Is a Novel Molecular Mechanism Linking Folate Availability and Cell Function.J Nutr. 2017 Jul;147(7):1237-1242. doi: 10.3945/jn.117.248823. Epub 2017 Jun 7. J Nutr. 2017. PMID: 28592519 Free PMC article. Review.
-
The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling.Nutrients. 2017 Oct 27;9(11):1176. doi: 10.3390/nu9111176. Nutrients. 2017. PMID: 29077002 Free PMC article. Review.
Cited by
-
Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance.Cancer Biol Med. 2014 Dec;11(4):255-63. doi: 10.7497/j.issn.2095-3941.2014.04.004. Cancer Biol Med. 2014. PMID: 25610711 Free PMC article. Review.
-
Regulation of Human Trophoblast GLUT3 Glucose Transporter by Mammalian Target of Rapamycin Signaling.Int J Mol Sci. 2015 Jun 16;16(6):13815-28. doi: 10.3390/ijms160613815. Int J Mol Sci. 2015. PMID: 26086828 Free PMC article.
-
Vasoactive Intestinal Peptide induces glucose and neutral amino acid uptake through mTOR signalling in human cytotrophoblast cells.Sci Rep. 2019 Nov 20;9(1):17152. doi: 10.1038/s41598-019-53676-3. Sci Rep. 2019. PMID: 31748639 Free PMC article.
-
Ruminant Placental Adaptation in Early Maternal Undernutrition: An Overview.Front Vet Sci. 2021 Oct 20;8:755034. doi: 10.3389/fvets.2021.755034. eCollection 2021. Front Vet Sci. 2021. PMID: 34746288 Free PMC article. Review.
-
Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway.Antioxidants (Basel). 2020 Feb 1;9(2):121. doi: 10.3390/antiox9020121. Antioxidants (Basel). 2020. PMID: 32024207 Free PMC article.
References
-
- Alessi DR, Pearce LR, García-Martínez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:1–4. - PubMed
-
- Ayuk PT-Y, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. - PubMed
-
- Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. The SNAT4 isoform of the system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol. 2006;290:C305–C312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

