The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes
- PMID: 23165896
- PMCID: PMC3569640
- DOI: 10.1002/emmm.201201846
The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes
Abstract
Immunoglobulins, antigens and complement can assemble to form immune complexes (IC). ICs can be detrimental as they propagate inflammation in autoimmune diseases. Like ICs, submicron extracellular vesicles termed microparticles (MP) are present in the synovial fluid from patients affected with autoimmune arthritis. We examined MPs in rheumatoid arthritis (RA) using high sensitivity flow cytometry and electron microscopy. We find that the MPs in RA synovial fluid are highly heterogeneous in size. The observed larger MPs were in fact MP-containing ICs (mpICs) and account for the majority of the detectable ICs. These mpICs frequently express the integrin CD41, consistent with platelet origin. Despite expression of the Fc receptor FcγRIIa by platelet-derived MPs, we find that the mpICs form independently of this receptor. Rather, mpICs display autoantigens vimentin and fibrinogen, and recognition of these targets by anti-citrullinated peptide antibodies contributes to the production of mpICs. Functionally, platelet mpICs are highly pro-inflammatory, eliciting leukotriene production by neutrophils. Taken together, our data suggest a unique role for platelet MPs as autoantigen-expressing elements capable of perpetuating formation of inflammatory ICs.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

A. Acquisition of fluorescent Sky Blue microspheres of 40–90 nm (mean = 90 nm, in cyan blue), 100–300 nm (mean = 220 nm, in pink), 400–600 nm (mean = 450 nm, in green), 700–900 nm (mean = 840 nm, in blue), 2500–4500 nm (mean = 3200 nm, in violet) diameter on a flow cytometer Canto II modified with a FSC-PMT small particles option. A scale bar ranging from 100 to 3200 nm based on the microsphere sizes (FSC-PMT) is presented and used to determine the relative dimensions of the MPs.
B,C. Representative FSC-PMT and SSC portrayals of the Annexin-V+ events detected in RA SF (B) and PA SF (C) revealing the dimension diversity of the MPs. The relative dimensions of the MPs are presented according to size-defined microsphere calibrations. Two major subpopulations (subpop 1 and 2) are detected in RA SF (B) while only one (subpop 1) is observed in PA SF (C) are identified on the graphs.
D. Triton sensitivity of the MPs in RA SF detected using Annexin-V labeling presented as % of untreated (control).
E. Flow cytometric quantifications of the Annexin-V+ MPs contained in RA and PA SF (n = 23 RA and n = 18 PA ***p = 0.0004). Statistical analyses are presented under the graph. Data are mean ± SEM.

A,B. Representative observations obtained using RA SF in which MPs [white boxes labelled MP (MP 1 and MP 2 (A) and MP (B)] were colocalized with ∼2 µm diameter mpICs. White arrows indicate the edge of the IC where MPs were detected. Black arrows indicate the carbon matrix of the perforated support film used for TEM. Insets (bottom panels) include individual MPs. Immunoglobulins detected using protein A conjugated nanospheres (10 nm) are indicated with white arrowheads and MPs detected using Annexin-V conjugated nanospheres (4 nm) are indicated by black arrowheads. Note that some MPs were Annexin-V negative.
C. Relatively smaller mpICs were also visualized. White and black arrowheads indicate immunoglobulins and phosphatidylserine, respectively. Scale bars are presented under each panel.

FSC-PMT and SSC dot plots of representative RA SF labelled with a combination of FITC-conjugated Annexin-V and Cy5-conjugated anti-IgG to demonstrate the presence of IgG on surface of Annexin-V+ MPs. The four-quadrant gates were positioned according to the isotypic controls. The MPs (Annexin-V+-IgG−) in blue show dimensions ranging mostly from 100 to 300 nm (lower inset) while the mpICs (Annexin-V+-IgG+) in green have dimensions from 700 to 3000 nm (upper inset). The relative diameters are presented according to size-defined microsphere calibrations.
Quantifications of the mpICs contained in RA and PA SF (n = 23 RA and n = 18 PA ***p < 0.0001).
Quantifications of the mpICs relative to the total amounts of detectable ICs in RA SF (n = 23). The statistical analyses are presented under each graph. Data are mean ± SEM.
FSC-PMT and SSC dot plots of representative PA SF labelled with a combination of FITC-conjugated Annexin-V and Cy5-conjugated anti-IgG.
FSC-PMT and SSC dot plots of RA SF patients labelled with PE-conjugated anti-CD41 and Cy5-conjugated anti-IgG. The four-quadrant gates were positioned according to the isotypic controls. The presence of CD41+ mpICs is evidenced by the dual expression of CD41 and IgG by MPs (green region). RA SF from two patients are presented to illustrate the heterogeneity that exists among the patients.
Quantifications of the CD41+ mpICs in RA and PA SF (n = 25 RA and n = 18 PA ***p = 0.0006). The statistical analyses are presented under each graph. Data are mean ± SEM.
Triton sensitivity of the CD41+ mpICs contained in RA SF presented as % of untreated (control) CD41+ mpICs.

A,B. The expression of CD32a by platelet MPs was determined by hs-FCM and Western blot analysis (inset in A). The platelet MPs were generated in vitro upon platelet GPVI stimulation (A) and the endogenous MPs in RA SF were detected using Annexin-V (B). Blue: isotype; grey: specific CD32a labeling (n = 3).
C. FSC-PMT and SSC profiles of the fluorescent platelets (top panel) and platelet MPs (bottom panel) (n = 10). The relative dimensions are presented according to size-defined microsphere calibrations.
D–G. Results obtained following the incubation of exogenous fluorescent platelet MPs in RA and PA SF (n = 10 RA and n = 18 PA). (D) Representative FSC-PMT and SSC plots obtained using RA (top) and PA SF (bottom). Two subpopulations (subpop) (1 and 2) were detected in RA and only one in PA SF (1). (E,F) hs-FCM evaluation of the presence of IgG on surface of MPs upon incubation in RA (E) and PA SF (F) using Cy5-conjugated anti-IgG antibody. The four-quadrant gates were positioned according to the isotypic controls. (E) MPs (in green) are IgG− and show dimensions ranging from 100 to 300 nm (lower inset) while the mpICs (in blue) have dimensions ranging from 700 to 3000 nm (upper inset). (D,E) The relative dimensions are presented according to size-defined microsphere calibrations. (G) hs-FCM quantifications of the mpICs were determined in absence (1st and 3rd column) or presence (2nd column) of CD32a blocking antibody (1st and 2nd column p = 0.4478 (unpaired t-test), 1st and 3rd column ***p < 0.0001). Statistical analyses are presented under the graphs. Data are mean ± SEM.
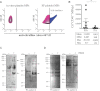
A,B The presence of citrullinated epitopes on platelet MPs was determined by hs-FCM using anti-citrulline antibody and its corresponding secondary antibody conjugated to Alexa 647. MPs were confirmed of platelet origin using a PE-conjugated anti-CD41 antibody. (A) The presence of citrullinated antigens on platelet MPs was determined in in vitro platelet MPs (left panel) and MPs from RA SF (right panel). The vertical lines were positioned according to the isotypic controls. Pink: Negative population; blue: Positive population. The % of positive platelet MPs is indicated on graphs. (B) Quantification of the citrulline+ CD41+ MPs in RA and PA SF (n = 8 RA and n = 8 PA *p = 0.0148).
C,D The proteins from platelet MPs obtained in vitro left unmodified (−PAD4) or citrullinated (+PAD4) were separated on SDS–PAGE and transferred to membranes. The membranes were incubated in presence of RA (C) or PA (D) SF and the recognition of MP-derived autoantigens by autoantibodies from SF was revealed using HRP-conjugated anti-human IgG. Arrows indicate proteins recognized by IgG from SF uniquely when the MPs were citrullinated. Two RA and PA SF are presented to illustrate the existing variability between patients (n = 12 RA and n = 8 PA).

A. Identification of the autoantibodies contained in CD41+ mpICs in RA SF. The binding specificity of the mpIC-eluted IgG to 40 antigens was determined on a Bio-Plex™ bead-based antigen array and only the antigens that presented significant binding are presented (n = 23 RA patients, identified 1–23). Colours denote amount of binding: undetectable (blue); progressively greater binding (green, yellow, red). Color scale is presented on right. The negative control (C) was obtained by incubating isotypic antibodies in SF. Cfc; cyclic citrullinated filaggrin, H2A/a; histone H2A/a.
B–D. The presence of autoantigens was examined on in vitro platelet MPs (left) and platelet MPs from RA SF (right) by hs-FCM (n = 4). (B) MPs were incubated with anti-vimentin and the interaction was revealed using Alexa 647-conjugated secondary antibody. (C) MPs were incubated with exogenous Alexa 647-fluorescent fibrinogen and associations quantified by hs-FCM. (D) MPs were incubated with anti-fibrinogen and the interaction with endogenous fibrinogen was determined using Alexa 647-conjugated secondary antibody. The vertical lines were positioned according to the isotypic controls. Green: negative population; blue: positive population. The % of positive MPs is indicated on graphs.
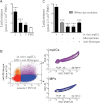
Quantifications of the leukotriene biosynthesis by neutrophils incubated in presence of RA SF mpICs [n = 3 distinct patients ***p < 0.0001 patients 1 and 2; **p = 0.0011 patient 3 (unpaired t-test, relative to PBS control)]. Data are mean ± SEM. Leukotrienes measured represent the sum of LTB4, 6-trans-LTB4, 12-epi-6-trans-LTB4, 20-OH-LTB4 and 20-COOH-LTB4.
Platelet mpICs form promptly upon co-incubation of platelet MPs with anti-fibrinogen in vitro. Representative FSC-PMT and SSC dot plot of mpICs. The presence of mpICs formed in vitro was demonstrated using a combination of FITC-conjugated Annexin-V and Alexa 647-conjugated secondary antibody. The four-quadrant gates were positioned according to the isotypic controls. The MPs (Annexin-V+-IgG−) presented in blue show dimensions ranging mostly from 100 to 500 nm (lower inset) while the mpICs (Annexin-V+-IgG+) presented in purple display dimensions ranging from 800 to 3000 nm (upper inset). The relative dimensions are presented according to size-defined microsphere calibrations.
Quantifications of the leukotriene biosynthesis by neutrophils incubated in presence of in vitro mpICs, anti-fibrinogen and platelet MPs. The stimulatory potency of mpICs compared to the others conditions was statistically significant [n = 3 ***p ≤ 0.0001 (unpaired t-test)]. Data are mean ± SEM. Leukotrienes measured represent the sum of LTB4, 6-trans-LTB4, 12-epi-6-trans-LTB4, 20-OH-LTB4 and 20-COOH-LTB4.
Similar articles
-
Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins.Arthritis Res Ther. 2010;12(4):R132. doi: 10.1186/ar3070. Epub 2010 Jul 7. Arthritis Res Ther. 2010. PMID: 20609218 Free PMC article.
-
Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells.Arthritis Res Ther. 2006;8(3):R64. doi: 10.1186/ar1926. Epub 2006 Mar 28. Arthritis Res Ther. 2006. PMID: 16569263 Free PMC article.
-
Anti-Fc gamma receptor III autoantibody is associated with soluble receptor in rheumatoid arthritis serum and synovial fluid.J Autoimmun. 1995 Apr;8(2):249-65. doi: 10.1006/jaut.1995.0019. J Autoimmun. 1995. PMID: 7612151
-
Circulating microparticles in systemic Lupus Erythematosus.Dan Med J. 2012 Nov;59(11):B4548. Dan Med J. 2012. PMID: 23171755 Review.
-
Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment.Autoimmun Rev. 2014 Nov;13(11):1114-20. doi: 10.1016/j.autrev.2014.08.012. Epub 2014 Aug 23. Autoimmun Rev. 2014. PMID: 25182207 Review.
Cited by
-
On the cellular metabolism of the click chemistry probe 19-alkyne arachidonic acid.J Lipid Res. 2016 Oct;57(10):1821-1830. doi: 10.1194/jlr.M067637. Epub 2016 Aug 18. J Lipid Res. 2016. PMID: 27538823 Free PMC article.
-
The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis.Cells. 2023 Nov 27;12(23):2716. doi: 10.3390/cells12232716. Cells. 2023. PMID: 38067147 Free PMC article. Review.
-
Development of Artificial Plasma Membranes Derived Nanovesicles Suitable for Drugs Encapsulation.Cells. 2020 Jul 6;9(7):1626. doi: 10.3390/cells9071626. Cells. 2020. PMID: 32640653 Free PMC article.
-
Detection and quantification of microparticles from different cellular lineages using flow cytometry. Evaluation of the impact of secreted phospholipase A2 on microparticle assessment.PLoS One. 2015 Jan 14;10(1):e0116812. doi: 10.1371/journal.pone.0116812. eCollection 2015. PLoS One. 2015. PMID: 25587983 Free PMC article.
-
Extracellular Vesicles as Potential Therapeutics for Inflammatory Diseases.Int J Mol Sci. 2021 May 22;22(11):5487. doi: 10.3390/ijms22115487. Int J Mol Sci. 2021. PMID: 34067503 Free PMC article. Review.
References
-
- Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–783. - PubMed
-
- Berckmans RJ, Nieuwland R, Tak PP, Boing AN, Romijn FP, Kraan MC, Breedveld FC, Hack CE, Sturk A. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857–2866. - PubMed
-
- Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

