Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature
- PMID: 23165928
- PMCID: PMC3954855
- DOI: 10.1039/c2np20085f
Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature
Abstract
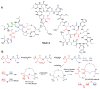
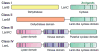
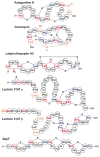
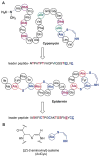
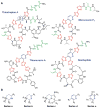
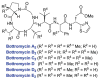
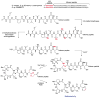
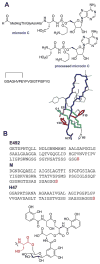
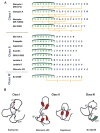
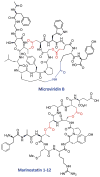
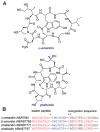
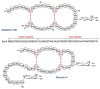
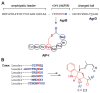
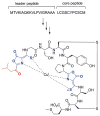
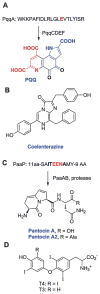
This review presents recommended nomenclature for the biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPPs), a rapidly growing class of natural products. The current knowledge regarding the biosynthesis of the >20 distinct compound classes is also reviewed, and commonalities are discussed.
Figures

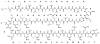





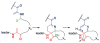






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

