Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity
- PMID: 23165940
- PMCID: PMC3573034
- DOI: 10.3892/mmr.2012.1189
Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity
Abstract
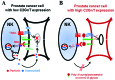
Core2 β-1,6-N-acetylglucosaminyltransferase (C2GnT) forms an N-acetylglucosamine branch in the O-glycans (core2 O-glycans) of cell surface glycoproteins. We previously revealed that the expression of C2GnT is positively correlated with poor prognosis in prostate cancer patients. However, the detailed mechanisms underlying their poor prognosis remain unclear. In the current study, we report that the core2 O-glycans carried by the surface MUC1 glycoproteins of prostate cancer cells play an important role in the evasion of NK cell immunity. In C2GnT‑expressing prostate cancer cells, the MUC1 core2 O-glycans are modified with poly-N-acetyllactosamine. MUC1 glycoproteins carrying poly-N-acetyllactosamine attenuated the interaction of the cancer cells with NK cells, resulting in decreased secretion of granzyme B by the NK cells. Poly‑N‑acetyllactosamine also interfered with the ability of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to access the cancer cell surface. These effects of poly-N-acetyllactosamine on NK cells render C2GnT-expressing prostate cancer cells resistant to NK cell cytotoxicity. By contrast, C2GnT-deficient prostate cancer cells carrying a lower amount of poly-N-acetyllactosamine than the C2GnT-expressing prostate cancer cells were significantly more susceptible to NK cell cytotoxicity. Our results strongly suggest that C2GnT-expressing prostate cancer cells evade NK cell immunity and survive longer in the host blood circulation, thereby resulting in the promotion of prostate cancer metastasis.
Figures




Similar articles
-
MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis.Int J Oncol. 2012 Jun;40(6):1831-8. doi: 10.3892/ijo.2012.1411. Epub 2012 Mar 23. Int J Oncol. 2012. PMID: 22446589 Free PMC article.
-
A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans.EMBO J. 2011 Jun 28;30(15):3173-85. doi: 10.1038/emboj.2011.215. EMBO J. 2011. PMID: 21712812 Free PMC article.
-
Core 2 β1,6-N-acetylglucosaminyltransferases accelerate the escape of choriocarcinoma from natural killer cell immunity.Biochem Biophys Rep. 2021 Feb 13;26:100951. doi: 10.1016/j.bbrep.2021.100951. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33644424 Free PMC article.
-
Tumor defense systems using O-glycans.Biol Pharm Bull. 2012;35(10):1633-6. doi: 10.1248/bpb.b12-00367. Biol Pharm Bull. 2012. PMID: 23037152 Review.
-
Two opposing roles of O-glycans in tumor metastasis.Trends Mol Med. 2012 Apr;18(4):224-32. doi: 10.1016/j.molmed.2012.02.001. Epub 2012 Mar 16. Trends Mol Med. 2012. PMID: 22425488 Free PMC article. Review.
Cited by
-
Could Harnessing Natural Killer Cell Activity Be a Promising Therapy for Prostate Cancer?Crit Rev Immunol. 2021;41(2):101-106. doi: 10.1615/CritRevImmunol.2021037614. Crit Rev Immunol. 2021. PMID: 34348004 Free PMC article. Review.
-
Detection of Core2 β-1,6-N-Acetylglucosaminyltransferase in Post-Digital Rectal Examination Urine Is a Reliable Indicator for Extracapsular Extension of Prostate Cancer.PLoS One. 2015 Sep 21;10(9):e0138520. doi: 10.1371/journal.pone.0138520. eCollection 2015. PLoS One. 2015. PMID: 26390303 Free PMC article.
-
Mucins in pancreatic cancer and its microenvironment.Nat Rev Gastroenterol Hepatol. 2013 Oct;10(10):607-20. doi: 10.1038/nrgastro.2013.120. Epub 2013 Jul 16. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23856888 Free PMC article. Review.
-
Tumor-Associated Glycans and Immune Surveillance.Vaccines (Basel). 2013 Jun 17;1(2):174-203. doi: 10.3390/vaccines1020174. Vaccines (Basel). 2013. PMID: 26343966 Free PMC article. Review.
-
Clinical Characteristics, Prognostic Factors and Therapeutic Strategies in Gastric Cancer Patients With Bone Metastasis: A Retrospective Analysis.Cancer Med. 2025 Mar;14(6):e70781. doi: 10.1002/cam4.70781. Cancer Med. 2025. PMID: 40105370 Free PMC article.
References
-
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
-
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. - PubMed
-
- Yousefi S, Higgins E, Daoling Z, Pollex-Krüger A, Hindsgaul O, Dennis JW. Increased UDP-GlcNAc:Gal beta 1–3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991;266:1772–1782. - PubMed
-
- Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. - PubMed
-
- Machida E, Nakayama J, Amano J, Fukuda M. Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 2001;61:2226–2231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

