Real-time cell viability assays using a new anthracycline derivative DRAQ7®
- PMID: 23165976
- PMCID: PMC3558543
- DOI: 10.1002/cyto.a.22228
Real-time cell viability assays using a new anthracycline derivative DRAQ7®
Abstract
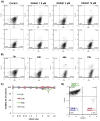
The exclusion of charged fluorescent dyes by intact cells has become a well-established assay for determining viability of cells. In search for a noninvasive fluorescent probe capable of long-term monitoring of cell death in real-time, we evaluated a new anthracycline derivative DRAQ7. The novel probe does not penetrate the plasma membrane of living cells but when the membrane integrity is compromised, it enters and binds readily to nuclear DNA to report cell death. It proved to be nontoxic to a panel of cancer cell lines grown continuously for up to 72 h and did not induce any detectable DNA damage signaling when analyzed using laser scanning microscopy and flow cytometry. The DRAQ7 provided a sensitive, real-time readout of cell death induced by a variety of stressors such as hypoxia, starvation, and drug-induced cytotoxicity. The overall responses to anticancer agents and resulting pharmacological dose-response profiles were not affected by the growth of tumor cells in the presence DRAQ7. Moreover, we for the first time introduced a near real-time microflow cytometric assay based on combination of DRAQ7 and mitochondrial inner membrane potential (ΔΨ(m) ) sensitive probe TMRM. We provide evidence that this low-dosage, real-time labeling procedure provides multiparameter and kinetic fingerprint of anticancer drug action.
Copyright © 2012 International Society for Advancement of Cytometry.
Figures



References
-
- Wlodkowic D, Skommer J, Pelkonen J. Multiparametric analysis of HA14-1-induced apoptosis in follicular lymphoma cells. Leuk Res. 2006;30:1187–92. - PubMed
-
- Wlodkowic D, Skommer J, Pelkonen J. Brefeldin A triggers apoptosis associated with mitochondrial breach and enhances HA14-1- and anti-Fas-mediated cell killing in follicular lymphoma cells. Leuk Res. 2007;31:1687–700. - PubMed
-
- Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. - PubMed
-
- Khoshmanesh K, Akagi J, Nahavandi S, Skommer J, Baratchi S, Cooper JM, Kalantar-Zadeh K, Williams DE, Wlodkowic D. Dynamic analysis of drug-induced cytotoxicity using chip-based dielectrophoretic cell immobilization technology. Anal Chem. 2011;83:2133–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

