Inhibiting periapical lesions through AAV-RNAi silencing of cathepsin K
- PMID: 23166044
- PMCID: PMC3545691
- DOI: 10.1177/0022034512468757
Inhibiting periapical lesions through AAV-RNAi silencing of cathepsin K
Abstract
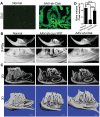
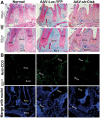
Dental caries, one of the most prevalent infectious diseases worldwide, affects approximately 80% of children and the majority of adults. Dental caries may result in endodontic disease, leading to dental pulp necrosis, periapical inflammation and bone resorption, severe pain, and tooth loss. Periapical inflammation may also increase inflammation in other parts of the body. Although many studies have attempted to develop therapies for this disease, there is still an urgent need for effective treatments. In this study, we applied a novel gene therapeutic approach using recombinant adeno-associated virus (AAV)-mediated RNAi knockdown of Cathepsin K (Ctsk) gene expression, to target osteoclasts and periapical bone resorption in a mouse model. We found that AAV-sh-Cathepsin K (AAV-sh-Ctsk) impaired osteoclast function in vivo and furthermore reduced bacterial infection-stimulated bone resorption by 88%. Reduced periapical lesion size was accompanied by decreases in mononuclear leukocyte infiltration and inflammatory cytokine expression. Our study shows that AAV-RNAi silencing of Cathepsin K in periapical tissues can significantly reduce endodontic disease development, bone destruction, and inflammation in the periapical lesion. This is the first demonstration that AAV-mediated RNAi knockdown gene therapy may significantly reduce the severity of endodontic disease.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


Similar articles
-
Silencing of Ac45 Simultaneously Inhibits Osteoclast-Mediated Bone Resorption and Attenuates Dendritic Cell-Mediated Inflammation through Impairing Acidification and Cathepsin K Secretion.Infect Immun. 2020 Dec 15;89(1):e00436-20. doi: 10.1128/IAI.00436-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33077625 Free PMC article.
-
The silencing of cathepsin K used in gene therapy for periodontal disease reveals the role of cathepsin K in chronic infection and inflammation.J Periodontal Res. 2016 Oct;51(5):647-60. doi: 10.1111/jre.12345. Epub 2016 Jan 11. J Periodontal Res. 2016. PMID: 26754272 Free PMC article.
-
RNA interference-mediated silencing of Atp6i prevents both periapical bone erosion and inflammation in the mouse model of endodontic disease.Infect Immun. 2013 Apr;81(4):1021-30. doi: 10.1128/IAI.00756-12. Infect Immun. 2013. PMID: 23166162 Free PMC article.
-
Recombinant AAV as a platform for translating the therapeutic potential of RNA interference.Mol Ther. 2014 Apr;22(4):692-701. doi: 10.1038/mt.2013.285. Epub 2013 Dec 19. Mol Ther. 2014. PMID: 24352214 Free PMC article. Review.
-
The role of cathepsin K in oral and maxillofacial disorders.Oral Dis. 2016 Mar;22(2):109-15. doi: 10.1111/odi.12378. Epub 2015 Nov 23. Oral Dis. 2016. PMID: 26458004 Review.
Cited by
-
Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway.Cell Prolif. 2021 Jul;54(7):e13058. doi: 10.1111/cpr.13058. Epub 2021 May 30. Cell Prolif. 2021. PMID: 34053135 Free PMC article.
-
A small molecule, odanacatib, inhibits inflammation and bone loss caused by endodontic disease.Infect Immun. 2015 Apr;83(4):1235-45. doi: 10.1128/IAI.01713-14. Epub 2015 Jan 12. Infect Immun. 2015. PMID: 25583522 Free PMC article.
-
Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice.J Bone Miner Res. 2013 Jul;28(7):1631-40. doi: 10.1002/jbmr.1894. J Bone Miner Res. 2013. PMID: 23426919 Free PMC article.
-
Targeting Atp6v1c1 Prevents Inflammation and Bone Erosion Caused by Periodontitis and Reveals Its Critical Function in Osteoimmunology.PLoS One. 2015 Aug 14;10(8):e0134903. doi: 10.1371/journal.pone.0134903. eCollection 2015. PLoS One. 2015. PMID: 26274612 Free PMC article.
-
C/ebpα controls osteoclast terminal differentiation, activation, function, and postnatal bone homeostasis through direct regulation of Nfatc1.J Pathol. 2018 Mar;244(3):271-282. doi: 10.1002/path.5001. Epub 2018 Jan 29. J Pathol. 2018. PMID: 29083488 Free PMC article.
References
-
- Asagiri M, Hirai T, Kunigami T, Kamano S, Gober HJ, Okamoto K, et al. (2008). Cathepsin K-dependent Toll-like receptor 9 signaling revealed in experimental arthritis. Science 319:624-627. - PubMed
-
- Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, et al. (2010). IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther 17:567-576. - PubMed
-
- Carter BJ. (2005). Adeno-associated virus vectors in clinical trials. Hum Gene Ther 16:541-550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

