The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination
- PMID: 23166151
- PMCID: PMC3540992
- DOI: 10.1158/2159-8290.CD-12-0329
The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination
Abstract
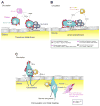
Tumor cells transit from the primary tumor via the blood circulation to form metastases in distant organs. During this process, tumor cells encounter a number of environmental challenges and stimuli that profoundly impact their metastatic potential. Here, we review the cooperative and dynamic host-tumor cell interactions that support and promote the hematogenous dissemination of cancer cells to sites of distant metastasis. In particular, we discuss what is known about the cross-talk occurring among tumor cells, platelets, leukocytes, and endothelial cells and how these cell-cell interactions are organized both temporally and spatially at sites of extravasation and in the early metastatic niche.
Significance: Metastasis is a function not only of tumor cells but also involves cooperative interactions of those cells with normal cells of the body, in particular platelets and leukocytes. These other cell types alter the behavior of the tumor cells themselves and of endothelial cells lining the vasculature and assist in tumor cell arrest and extravasation at sites of metastasis and subsequently in the establishment of tumor cells in the early metastatic niche. A better understanding of the important role that these contact and paracrine interactions play during metastasis will offer new opportunities for therapeutic intervention.
©2012 AACR.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

References
-
- Fidler IJ. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. - PubMed
-
- Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26:513–23. - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

