Interaction between the elastin peptide VGVAPG and human elastin binding protein
- PMID: 23166321
- PMCID: PMC3543015
- DOI: 10.1074/jbc.M112.419929
Interaction between the elastin peptide VGVAPG and human elastin binding protein
Abstract
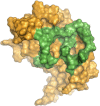
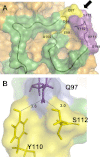
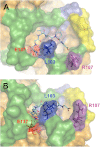
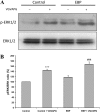
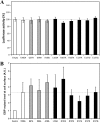
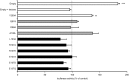
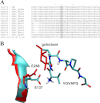
The elastin binding protein (EBP), a spliced variant of lysosomal β-galactosidase, is the primary receptor of elastin peptides that have been linked to emphysema, aneurysm and cancer progression. The sequences recognized by EBP share the XGXXPG consensus pattern found in numerous matrix proteins, notably in elastin where the VGVAPG motif is repeated. To delineate the elastin binding site of human EBP, we built a homology model of this protein and docked VGVAPG on its surface. Analysis of this model suggested that Gln-97 and Asp-98 were required for interaction with VGVAPG because they contribute to the definition of a pocket thought to represent the elastin binding site of EBP. Additionally, we proposed that Leu-103, Arg-107, and Glu-137 were essential residues because they could interact with VGVAPG itself. Site-directed mutagenesis experiments at these key positions validated our model. This work therefore provides the first structural data concerning the interaction of the VGVAPG with its cognate receptor. The present structural data should now allow the development of EBP-specific antagonists.
Figures
References
-
- Kielty C. M. (2006) Elastic fibres in health and disease. Expert Rev. Mol. Med. 8, 1–23 - PubMed
-
- Vrhovski B., Weiss A. S. (1998) Biochemistry of tropoelastin. Eur. J. Biochem. 258, 1–18 - PubMed
-
- Hinek A. (1994) Nature and the multiple functions of the 67-kD elastin-/laminin binding protein. Cell Adhes. Commun. 2, 185–193 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

