piRNA and spermatogenesis in mice
- PMID: 23166399
- PMCID: PMC3539364
- DOI: 10.1098/rstb.2011.0338
piRNA and spermatogenesis in mice
Abstract
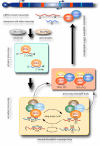
Transposable elements and their fossil sequences occupy about half of the genome in mammals. While most of these selfish mobile elements have been inactivated by truncations and mutations during evolution, some copies remain competent to transpose and/or amplify, posing an ongoing genetic threat. To control such mutagenic sequences, host genomes have developed multiple layers of defence mechanisms, including epigenetic regulation and RNA silencing. Germ cells, in particular, employ the piwi-small RNA pathway, which plays a central and adaptive role in safeguarding the germline genome from retrotransposons. Recent studies have revealed that a class of developmentally regulated genes, which have long been implicated in germ cell specification and differentiation, such as vasa and tudor family genes, play key roles in the piwi pathway to suppress retrotransposons, indicating that the piwi-mediated genome protection is at the core of germline development. Furthermore, while the piwi system primarily operates post-transcriptionally at the RNA level, it also affects the epigenetics of cognate genome loci, offering an intriguing link between small RNAs and transcriptional control in mammals. In this review, we summarize our current understanding of the piwi pathway in mice, which is emerging as a fundamental component of spermatogenesis that ensures male fertility and genome integrity.
Figures
References
-
- Gundry M, Vijg J. 2012. Direct mutation analysis by high-throughput sequencing: from germline to low-abundant, somatic variants. Mutat. Res. 729, 1–1510.1016/j.mrfmmm.2011.10.001 (doi:10.1016/j.mrfmmm.2011.10.001) - DOI - DOI - PMC - PubMed
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. 2005. DNA repair and mutagenesis, 2nd edn. Washington, DC: ASM Press
-
- Kazazian HH, Jr, 2004. Mobile elements: drivers of genome evolution. Science 303, 1626–163210.1126/science.1089670 (doi:10.1126/science.1089670) - DOI - DOI - PubMed
-
- Matsui T, Leung D, Miyashita H, Maksakova IA, Miyachi H, Kimura H, Tachibana M, Lorincz MC, Shinkai Y. 2010. Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature 464, 927–93110.1038/nature08858 (doi:10.1038/nature08858) - DOI - DOI - PubMed
-
- Rowe HM, et al. 2010. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463, 237–24010.1038/nature08674 (doi:10.1038/nature08674) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
