A large animal neuropathic pain model in sheep: a strategy for improving the predictability of preclinical models for therapeutic development
- PMID: 23166445
- PMCID: PMC3500921
- DOI: 10.2147/JPR.S34977
A large animal neuropathic pain model in sheep: a strategy for improving the predictability of preclinical models for therapeutic development
Abstract
Background: Evaluation of analgesics in large animals is a necessary step in the development of better pain medications or gene therapy prior to clinical trials. However, chronic neuropathic pain models in large animals are limited. To address this deficiency, we developed a neuropathic pain model in sheep, which shares many anatomical similarities in spine dimensions and cerebrospinal fluid volume as humans.
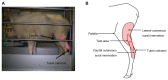
Methods: A neuropathic pain state was induced in sheep by tight ligation and axotomy of the common peroneal nerve. The analgesic effect of intrathecal (IT) morphine was investigated. Interspecies comparison was conducted by analyzing the ceiling doses of IT morphine for humans, sheep, and rats.
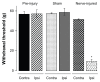
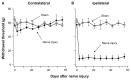
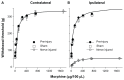
Results: Peroneal nerve injury (PNI) produced an 86% decrease in von-Frey filament-evoked withdrawal threshold on postsurgery day 3 and the decrease lasted for the 8-week test period. Compared to the pre-injury, sham, and contralateral hindlimb, the IT morphine dose that produces 50% of maximum analgesia (ED(50)) for injured PNI hindlimb was 1.8-fold larger and E(max), the dose that produces maximal analgesia, was 6.1-fold lower. The sheep model closely predicts human IT morphine ceiling dose by allometric scaling. This is in contrast to the approximately 10-fold lower morphine ceiling dose predicted by the rat spinal nerve ligated or spared nerve injury models.
Conclusion: PNI sheep model has a fast onset and shows stable and long-lasting pain behavioral characteristics. Since the antinociceptive properties of IT morphine are similar to those observed in humans, the PNI sheep model will be a useful tool for the development of analgesics. Its large size and consistent chronic pain behavior will facilitate the development and evaluation of surgical intervention and gene therapy. The PNI sheep pain model provides us with the opportunity for multi-species testing, which will improve the success of clinical trials.
Keywords: interspecies drug scaling; neuropathic pain model; ovine; ovine pain model.
Figures

Similar articles
-
Synergistic antinociceptive interactions of morphine and clonidine in rats with nerve-ligation injury.Anesthesiology. 1997 Jan;86(1):196-204. doi: 10.1097/00000542-199701000-00024. Anesthesiology. 1997. PMID: 9009955
-
Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats.Pain. 1997 May;71(1):57-64. doi: 10.1016/s0304-3959(97)03337-x. Pain. 1997. PMID: 9200174
-
The effectiveness of spinal and systemic morphine on rat dorsal horn neuronal responses in the spinal nerve ligation model of neuropathic pain.Pain. 1999 Mar;80(1-2):215-28. doi: 10.1016/s0304-3959(98)00208-5. Pain. 1999. PMID: 10204734
-
Sheep Models in Translational Surgery.Eur Surg Res. 2025;66(1):39-45. doi: 10.1159/000546157. Epub 2025 Apr 26. Eur Surg Res. 2025. PMID: 40288355 Review.
-
Preclinical Neuropathic Pain Assessment; the Importance of Translatability and Bidirectional Research.Front Pharmacol. 2021 Feb 8;11:614990. doi: 10.3389/fphar.2020.614990. eCollection 2020. Front Pharmacol. 2021. PMID: 33628181 Free PMC article. Review.
Cited by
-
Small Ruminants and Its Use in Regenerative Medicine: Recent Works and Future Perspectives.Biology (Basel). 2021 Mar 22;10(3):249. doi: 10.3390/biology10030249. Biology (Basel). 2021. PMID: 33810087 Free PMC article. Review.
-
The Sheep as a Large Animal Model for the Investigation and Treatment of Human Disorders.Biology (Basel). 2022 Aug 23;11(9):1251. doi: 10.3390/biology11091251. Biology (Basel). 2022. PMID: 36138730 Free PMC article. Review.
-
Ovine model of neuropathic pain for assessing mechanisms of spinal cord stimulation therapy via dorsal horn recordings, von Frey filaments, and gait analysis.J Pain Res. 2018 Jun 15;11:1147-1162. doi: 10.2147/JPR.S139843. eCollection 2018. J Pain Res. 2018. PMID: 29942150 Free PMC article.
-
Pain assessment in animal models: do we need further studies?J Pain Res. 2014 May 8;7:227-36. doi: 10.2147/JPR.S59161. eCollection 2014. J Pain Res. 2014. PMID: 24855386 Free PMC article. Review.
-
Validation of the Unesp-Botucatu composite scale to assess acute postoperative abdominal pain in sheep (USAPS).PLoS One. 2020 Oct 14;15(10):e0239622. doi: 10.1371/journal.pone.0239622. eCollection 2020. PLoS One. 2020. PMID: 33052903 Free PMC article.
References
-
- Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. - PubMed
-
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. - PubMed
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. - PubMed
-
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. - PubMed
-
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

