Sensorimotor learning biases choice behavior: a learning neural field model for decision making
- PMID: 23166483
- PMCID: PMC3499253
- DOI: 10.1371/journal.pcbi.1002774
Sensorimotor learning biases choice behavior: a learning neural field model for decision making
Abstract
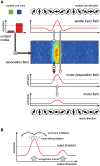
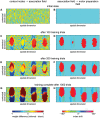
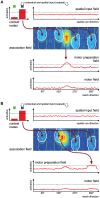
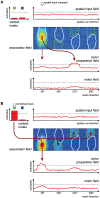
According to a prominent view of sensorimotor processing in primates, selection and specification of possible actions are not sequential operations. Rather, a decision for an action emerges from competition between different movement plans, which are specified and selected in parallel. For action choices which are based on ambiguous sensory input, the frontoparietal sensorimotor areas are considered part of the common underlying neural substrate for selection and specification of action. These areas have been shown capable of encoding alternative spatial motor goals in parallel during movement planning, and show signatures of competitive value-based selection among these goals. Since the same network is also involved in learning sensorimotor associations, competitive action selection (decision making) should not only be driven by the sensory evidence and expected reward in favor of either action, but also by the subject's learning history of different sensorimotor associations. Previous computational models of competitive neural decision making used predefined associations between sensory input and corresponding motor output. Such hard-wiring does not allow modeling of how decisions are influenced by sensorimotor learning or by changing reward contingencies. We present a dynamic neural field model which learns arbitrary sensorimotor associations with a reward-driven Hebbian learning algorithm. We show that the model accurately simulates the dynamics of action selection with different reward contingencies, as observed in monkey cortical recordings, and that it correctly predicted the pattern of choice errors in a control experiment. With our adaptive model we demonstrate how network plasticity, which is required for association learning and adaptation to new reward contingencies, can influence choice behavior. The field model provides an integrated and dynamic account for the operations of sensorimotor integration, working memory and action selection required for decision making in ambiguous choice situations.
Conflict of interest statement
The authors have declared that no competing interests exist.
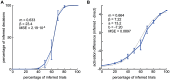
Figures




References
-
- Tversky A, Kahneman D (1981) The Framing of Decisions and the Psychology of Choice. Science 211: 453–458 doi:10.1126/science.7455683 - DOI - PubMed
-
- Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298. - PubMed
-
- Andersen RA, Cui H (2009) Intention, Action Planning, and Decision Making in Parietal-Frontal Circuits. Neuron 63: 568–583 doi:10.1016/j.neuron.2009.08.028 - DOI - PubMed
-
- Klaes C, Westendorff S, Chakrabarti S, Gail A (2011) Choosing Goals, Not Rules: Deciding among Rule-Based Action Plans. Neuron 70: 536–548 doi:10.1016/j.neuron.2011.02.053 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

