Lamin b1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice
- PMID: 23166514
- PMCID: PMC3499363
- DOI: 10.1371/journal.pgen.1003059
Lamin b1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice
Abstract
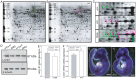
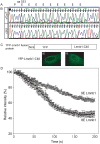
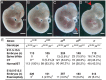
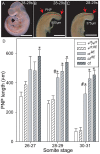
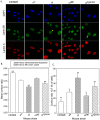
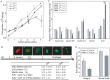
Neural tube defects (NTDs), including spina bifida and anencephaly, are common birth defects whose complex multigenic causation has hampered efforts to delineate their molecular basis. The effect of putative modifier genes in determining NTD susceptibility may be investigated in mouse models, particularly those that display partial penetrance such as curly tail, a strain in which NTDs result from a hypomorphic allele of the grainyhead-like-3 gene. Through proteomic analysis, we found that the curly tail genetic background harbours a polymorphic variant of lamin B1, lacking one of a series of nine glutamic acid residues. Lamins are intermediate filament proteins of the nuclear lamina with multiple functions that influence nuclear structure, cell cycle properties, and transcriptional regulation. Fluorescence loss in photobleaching showed that the variant lamin B1 exhibited reduced stability in the nuclear lamina. Genetic analysis demonstrated that the variant also affects neural tube closure: the frequency of spina bifida and anencephaly was reduced three-fold when wild-type lamin B1 was bred into the curly tail strain background. Cultured fibroblasts expressing variant lamin B1 show significantly increased nuclear dysmorphology and diminished proliferative capacity, as well as premature senescence, associated with reduced expression of cyclins and Smc2, and increased expression of p16. The cellular basis of spinal NTDs in curly tail embryos involves a proliferation defect localised to the hindgut epithelium, and S-phase progression was diminished in the hindgut of embryos expressing variant lamin B1. These observations indicate a mechanistic link between altered lamin B1 function, exacerbation of the Grhl3-mediated cell proliferation defect, and enhanced susceptibility to NTDs. We conclude that lamin B1 is a modifier gene of major effect for NTDs resulting from loss of Grhl3 function, a role that is likely mediated via the key function of lamin B1 in maintaining integrity of the nuclear envelope and ensuring normal cell cycle progression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Nadeau JH (2003) Modifier genes and protective alleles in humans and mice. Curr Opin Genet Dev 13: 290–295. - PubMed
-
- Bassuk AG, Kibar Z (2009) Genetic basis of neural tube defects. Semin Pediatr Neurol 16: 101–110. - PubMed
-
- Copp AJ, Greene NDE, Murdoch JN (2003) The genetic basis of mammalian neurulation. Nat Rev Genet 4: 784–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

