Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1
- PMID: 23166521
- PMCID: PMC3499365
- DOI: 10.1371/journal.pgen.1003078
Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1
Abstract
The poly(A)-binding protein nuclear 1 (PABPN1) is a ubiquitously expressed protein that is thought to function during mRNA poly(A) tail synthesis in the nucleus. Despite the predicted role of PABPN1 in mRNA polyadenylation, little is known about the impact of PABPN1 deficiency on human gene expression. Specifically, it remains unclear whether PABPN1 is required for general mRNA expression or for the regulation of specific transcripts. Using RNA sequencing (RNA-seq), we show here that the large majority of protein-coding genes express normal levels of mRNA in PABPN1-deficient cells, arguing that PABPN1 may not be required for the bulk of mRNA expression. Unexpectedly, and contrary to the view that PABPN1 functions exclusively at protein-coding genes, we identified a class of PABPN1-sensitive long noncoding RNAs (lncRNAs), the majority of which accumulated in conditions of PABPN1 deficiency. Using the spliced transcript produced from a snoRNA host gene as a model lncRNA, we show that PABPN1 promotes lncRNA turnover via a polyadenylation-dependent mechanism. PABPN1-sensitive lncRNAs are targeted by the exosome and the RNA helicase MTR4/SKIV2L2; yet, the polyadenylation activity of TRF4-2, a putative human TRAMP subunit, appears to be dispensable for PABPN1-dependent regulation. In addition to identifying a novel function for PABPN1 in lncRNA turnover, our results provide new insights into the post-transcriptional regulation of human lncRNAs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
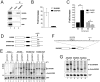
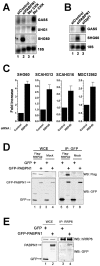
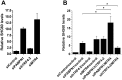
References
-
- Wahle E (1991) A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell 66: 759–768. - PubMed
-
- Kuhn U, Nemeth A, Meyer S, Wahle E (2003) The RNA binding domains of the nuclear poly(A)-binding protein. J Biol Chem 278: 16916–16925. - PubMed
-
- Kuhn U, Wahle E (2004) Structure and function of poly(A) binding proteins. Biochim Biophys Acta 1678: 67–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

