The efficacy of immediate diet for reducing local adverse events of inhaled corticosteroid: a pilot study
- PMID: 23166541
- PMCID: PMC3492379
- DOI: 10.4046/trd.2012.73.2.93
The efficacy of immediate diet for reducing local adverse events of inhaled corticosteroid: a pilot study
Abstract
Background: Local adverse events associated with inhaled corticosteroid use, including dysphonia, pharyngitis and oral candidiasis, can affect adherence for treatment. 'Mouth rinsing method' has been used for reducing local adverse events, but it cannot ensure complete prevention. The goal of this pilot study was to identify whether the 'immediate diet method' can reduce local adverse events in a limited number of patients.
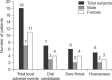
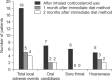
Methods: The study was conducted in a total of 98 patients, who had been prescribed a medium-dose fluticasone propionate for the first time, from January to October in 2010. One training nurse had performed the education on how to use the inhaler, including the mouth rinsing method. And with follow-ups at one month intervals, any patient who experienced such adverse events were educated on the immediate diet method, having a meal within 5 minutes after using an inhaler and they were checked on any incurrence of adverse events with one month intervals for 2 months.
Results: The mean age of patients was 65.9 years old. The local adverse events had incurred from 18.4% of the study subjects. When performed the follow-up observation in 18 patients with local adverse events after education on the immediate diet method, 14 patients (77.8%) had shown symptomatic improvements. Three of 4 patients did not show any improvement, in spite of implementing the immediate diet method. The other 1 patient did not practice the immediate diet method properly.
Conclusion: The immediate diet method may be useful in reducing the local adverse events, caused by the use of inhaled corticosteroid.
Keywords: Candidiasis, Oral; Drug Toxicity; Dysphonia; Pharyngitis; Steroids.
Figures
References
-
- Szefler SJ. Glucocorticoid therapy for asthma: clinical pharmacology. J Allergy Clin Immunol. 1991;88:147–165. - PubMed
-
- Hanania NA, Chapman KR, Kesten S. Adverse effects of inhaled corticosteroids. Am J Med. 1995;98:196–208. - PubMed
-
- Maxwell DL. Adverse effects of inhaled corticosteroids. Biomed Pharmacother. 1990;44:421–427. - PubMed
-
- Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126:213–219. - PubMed
-
- Toogood JH, Jennings B, Greenway RW, Chuang L. Candidiasis and dysphonia complicating beclomethasone treatment of asthma. J Allergy Clin Immunol. 1980;65:145–153. - PubMed

