Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature
- PMID: 23166608
- PMCID: PMC3499557
- DOI: 10.1371/journal.pone.0049163
Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature
Abstract
Background: Authors of randomized trial reports seem to hold a variety of views regarding the relationship between missing outcome data (MOD) and intention to treat (ITT). The objectives of this study were to systematically investigate how authors of methodology articles define ITT in the presence of MOD, how they recommend handling MOD under ITT, and to make a proposal for potential improvement in the definition and use of ITT in relation to MOD.
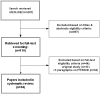
Methods and findings: We systematically searched MEDLINE in February 2009 for methodological articles written in English that devoted at least one paragraph to ITT and two other paragraphs to either ITT or MOD. We excluded original trial reports, observational studies, and clinical systematic reviews. Working in teams of two, we independently extracted relevant information from each eligible article. Of 1007 titles and abstracts reviewed, 66 articles met eligibility criteria. Five (8%) did not provide a definition of ITT; 25 (38%) mentioned MOD but did not discuss its relationship to ITT; and 36 (55%) discussed the relationship of MOD with ITT. These 36 articles described one or more of three statements: complete follow-up is required for ITT (58%); ITT and MOD are separate issues (17%); and ITT requires a specific strategy for handling MOD (78%); 17 (47%) endorsed more than one relationship. The most frequently mentioned strategies for handling MOD within ITT were: using the last outcome carried forward (50%); sensitivity analysis (50%); and use of available data to impute missing data (46%).
Conclusion: We found that there is no consensus on the definition of ITT in relation to MOD. For conceptual clarity, we suggest that both reports of randomized trials and systematic reviews separately consider and describe how they deal with participants with complete data and those with MOD.
Conflict of interest statement
Figures
References
-
- Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134: 657–662. - PubMed
-
- Food and Drug Administration (1988) Guideline for the format and content of the clinical and statistical sections of new drug applications. FDA, US Department of Health and Human Services Rockville, MA, USA.
-
- Nordic Council on Medicine (1989) Good clinical practice. Nordic Guidelines, NLN Publication No 28, Uppsala, Sweden.
-
- Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, et al... (1990) Intention to treat in clinical trials. In: Statistical Issues in Drug Research and Development (American Statistical Associations Group) Peace K E (ed) Marcel Dekker, New York: 331–350.
-
- Gravel J, Opatrny L, Shapiro S (2007) The intention-to-treat approach in randomized controlled trials: are authors saying what they do and doing what they say? Clin Trials 4: 350–356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous