Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.)
- PMID: 23166615
- PMCID: PMC3499563
- DOI: 10.1371/journal.pone.0049210
Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.)
Abstract
Background: Amino acid transporters (AATs) that transport amino acids across cellular membranes are essential for plant growth and development. To date, a genome-wide overview of the AAT gene family in rice is not yet available.
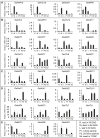
Methodology/principal findings: In this study, a total of 85 AAT genes were identified in rice genome and were classified into eleven distinct subfamilies based upon their sequence composition and phylogenetic relationship. A large number of OsAAT genes were expanded via gene duplication, 23 and 24 OsAAT genes were tandemly and segmentally duplicated, respectively. Comprehensive analyses were performed to investigate the expression profiles of OsAAT genes in various stages of vegetative and reproductive development by using data from EST, Microarrays, MPSS and Real-time PCR. Many OsAAT genes exhibited abundant and tissue-specific expression patterns. Moreover, 21 OsAAT genes were found to be differentially expressed under the treatments of abiotic stresses. Comparative analysis indicates that 26 AAT genes with close evolutionary relationships between rice and Arabidopsis exhibited similar expression patterns.
Conclusions/significance: This study will facilitate further studies on OsAAT family and provide useful clues for functional validation of OsAATs.
Conflict of interest statement
Figures







References
-
- Wipf D, Ludewig U, Tegeder M, Rentsch D, Koch W, et al. (2002) Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem Sci 27: 139–147. - PubMed
-
- Tegeder M (2012) Transporters for amino acids in plant cells: some functions and many unknowns. Curr Opin Plant Biol 15: 1–7. - PubMed
-
- Rentsch D, Schmidt S, Tegeder M (2007) Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett 581: 2281–2289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

