Conformational changes of blood ACE in chronic uremia
- PMID: 23166630
- PMCID: PMC3500299
- DOI: 10.1371/journal.pone.0049290
Conformational changes of blood ACE in chronic uremia
Abstract
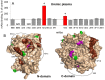
Background: The pattern of binding of monoclonal antibodies (mAbs) to 16 epitopes on human angiotensin I-converting enzyme (ACE) comprise a conformational ACE fingerprint and is a sensitive marker of subtle protein conformational changes.
Hypothesis: Toxic substances in the blood of patients with uremia due to End Stage Renal Disease (ESRD) can induce local conformational changes in the ACE protein globule and alter the efficacy of ACE inhibitors.
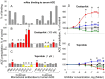
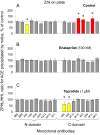
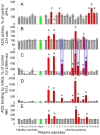
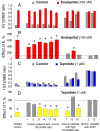
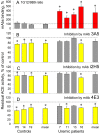
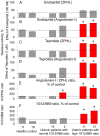
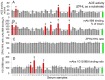
Methodology/principal findings: The recognition of ACE by 16 mAbs to the epitopes on the N and C domains of ACE was estimated using an immune-capture enzymatic plate precipitation assay. The precipitation pattern of blood ACE by a set of mAbs was substantially influenced by the presence of ACE inhibitors with the most dramatic local conformational change noted in the N-domain region recognized by mAb 1G12. The "short" ACE inhibitor enalaprilat (tripeptide analog) and "long" inhibitor teprotide (nonapeptide) produced strikingly different mAb 1G12 binding with enalaprilat strongly increasing mAb 1G12 binding and teprotide decreasing binding. Reduction in S-S bonds via glutathione and dithiothreitol treatment increased 1G12 binding to blood ACE in a manner comparable to enalaprilat. Some patients with uremia due to ESRD exhibited significantly increased mAb 1G12 binding to blood ACE and increased ACE activity towards angiotensin I accompanied by reduced ACE inhibition by inhibitory mAbs and ACE inhibitors.
Conclusions/significance: The estimation of relative mAb 1G12 binding to blood ACE detects a subpopulation of ESRD patients with conformationally changed ACE, which activity is less suppressible by ACE inhibitors. This parameter may potentially serve as a biomarker for those patients who may need higher concentrations of ACE inhibitors upon anti-hypertensive therapy.
Conflict of interest statement
Figures
Similar articles
-
Monoclonal Antibodies 1G12 and 6A12 to the N-domain of human angiotensin-converting enzyme: fine epitope mapping and antibody-based detection of ACE inhibitors in human blood.J Proteome Res. 2007 Apr;6(4):1580-94. doi: 10.1021/pr060658x. Epub 2007 Feb 28. J Proteome Res. 2007. PMID: 17326675
-
Fine epitope mapping of monoclonal antibody 5F1 reveals anticatalytic activity toward the N domain of human angiotensin-converting enzyme.Biochemistry. 2007 Aug 7;46(31):9019-31. doi: 10.1021/bi700489v. Epub 2007 Jul 14. Biochemistry. 2007. PMID: 17630779
-
Conformational fingerprint of blood and tissue ACEs: Personalized approach.PLoS One. 2018 Dec 27;13(12):e0209861. doi: 10.1371/journal.pone.0209861. eCollection 2018. PLoS One. 2018. PMID: 30589901 Free PMC article.
-
[Conformational Fingerprinting Using Monoclonal Antibodies <br />(on the Example of Angiotensin I-Converting Enzyme-ACE)].Mol Biol (Mosk). 2017 Nov-Dec;51(6):1046-1061. doi: 10.7868/S0026898417060155. Mol Biol (Mosk). 2017. PMID: 29271967 Review. Russian.
-
The design and properties of N-carboxyalkyldipeptide inhibitors of angiotensin-converting enzyme.Adv Enzymol Relat Areas Mol Biol. 1985;57:1-84. doi: 10.1002/9780470123034.ch1. Adv Enzymol Relat Areas Mol Biol. 1985. PMID: 2994404 Review.
Cited by
-
Tissue ACE phenotyping in lung cancer.PLoS One. 2019 Dec 26;14(12):e0226553. doi: 10.1371/journal.pone.0226553. eCollection 2019. PLoS One. 2019. PMID: 31877149 Free PMC article.
-
Human Angiotensin I-Converting Enzyme Produced by Different Cells: Classification of the SERS Spectra with Linear Discriminant Analysis.Biomedicines. 2022 Jun 12;10(6):1389. doi: 10.3390/biomedicines10061389. Biomedicines. 2022. PMID: 35740411 Free PMC article.
-
Predictive potential of ACE phenotyping in extrapulmonary sarcoidosis.Respir Res. 2022 Aug 22;23(1):211. doi: 10.1186/s12931-022-02145-z. Respir Res. 2022. PMID: 35996109 Free PMC article.
-
Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism.PLoS One. 2014 Jul 8;9(7):e102137. doi: 10.1371/journal.pone.0102137. eCollection 2014. PLoS One. 2014. PMID: 25003524 Free PMC article.
-
Blood ACE Phenotyping for Personalized Medicine: Revelation of Patients with Conformationally Altered ACE.Biomedicines. 2023 Feb 13;11(2):534. doi: 10.3390/biomedicines11020534. Biomedicines. 2023. PMID: 36831070 Free PMC article.
References
-
- Ehlers MWR, Riordan JF (1989) Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry 8: 5311–5318. - PubMed
-
- Bernstein KE, Xiao HD, Frenzel K, Li P, Shen XZ, et al. (2005) Six truisms concerning ACE and the renin-angiotensin system educed from the genetic analysis of mice. Circ Res 96: 1135–1144. - PubMed
-
- Sturrock ED, Anthony CS, Danilov SM (2012) Peptidyl-dipeptidase A/Angiotensin I-converting enzyme. In: Editors-in-Chief: Neil D. Rawlings, Guy Salvesen, Handbook of Proteolytic Enzymes, 3rd Edition, Academic Press, Oxford (in press).
-
- Caldwell PR, Seegal BC, Hsu KC, Das H, Soffer RL (1976) Angiotensin-converting enzyme: vascular endothelial localization. Science 191: 1050–1051. - PubMed
-
- Ryan US, Ryan JW, Whitaker C, Chiu A (1976) Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell 8: 124–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

