Effect of pullulan nanoparticle surface charges on HSA complexation and drug release behavior of HSA-bound nanoparticles
- PMID: 23166632
- PMCID: PMC3498137
- DOI: 10.1371/journal.pone.0049304
Effect of pullulan nanoparticle surface charges on HSA complexation and drug release behavior of HSA-bound nanoparticles
Abstract
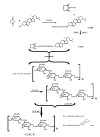
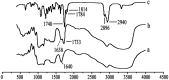
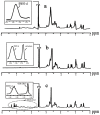
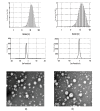
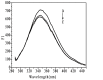
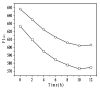
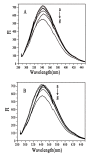
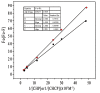
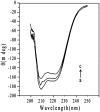
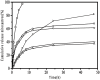
Nanoparticle (NP) compositions such as hydrophobicity and surface charge are vital to determine the presence and amount of human serum albumin (HSA) binding. The HSA binding influences drug release, biocompatibility, biodistribution, and intercellular trafficking of nanoparticles (NPs). Here, we prepared 2 kinds of nanomaterials to investigate HSA binding and evaluated drug release of HSA-bound NPs. Polysaccharides (pullulan) carboxyethylated to provide ionic derivatives were then conjugated to cholesterol groups to obtain cholesterol-modified carboxyethyl pullulan (CHCP). Cholesterol-modified pullulan (CHP) conjugate was synthesized with a similar degree of substitution of cholesterol moiety to CHCP. CHCP formed self-aggregated NPs in aqueous solution with a spherical structure and zeta potential of -19.9 ± 0.23 mV, in contrast to -1.21 ± 0.12 mV of CHP NPs. NPs could quench albumin fluorescence intensity with maximum emission intensity gradually decreasing up to a plateau at 9 to 12 h. Binding constants were 1.12 × 10(5) M(-1) and 0.70 × 10(5) M(-1) to CHP and CHCP, respectively, as determined by Stern-Volmer analysis. The complexation between HSA and NPs was a gradual process driven by hydrophobic force and inhibited by NP surface charge and shell-core structure. HSA conformation was altered by NPs with reduction of α-helical content, depending on interaction time and particle surface charges. These NPs could represent a sustained release carrier for mitoxantrone in vitro, and the bound HSA assisted in enhancing sustained drug release.
Conflict of interest statement
Figures

Similar articles
-
Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles.Nanomaterials (Basel). 2015 Dec 25;6(1):2. doi: 10.3390/nano6010002. Nanomaterials (Basel). 2015. PMID: 28344259 Free PMC article.
-
Pullulan-Based Nanoparticle-HSA Complex Formation and Drug Release Influenced by Surface Charge.Nanoscale Res Lett. 2018 Oct 10;13(1):317. doi: 10.1186/s11671-018-2729-5. Nanoscale Res Lett. 2018. PMID: 30306404 Free PMC article.
-
Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles.Nanomaterials (Basel). 2016 Sep 8;6(9):165. doi: 10.3390/nano6090165. Nanomaterials (Basel). 2016. PMID: 28335293 Free PMC article.
-
Synthesis and characterization of biotin modified cholesteryl pullulan as a novel anticancer drug carrier.Carbohydr Polym. 2014 Jan;99:720-7. doi: 10.1016/j.carbpol.2013.09.013. Epub 2013 Sep 10. Carbohydr Polym. 2014. PMID: 24274563
-
Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting.Carbohydr Polym. 2015 Jun 5;123:190-207. doi: 10.1016/j.carbpol.2015.01.032. Epub 2015 Feb 4. Carbohydr Polym. 2015. PMID: 25843851 Review.
Cited by
-
Novel Delivery of Mitoxantrone with Hydrophobically Modified Pullulan Nanoparticles to Inhibit Bladder Cancer Cell and the Effect of Nano-drug Size on Inhibition Efficiency.Nanoscale Res Lett. 2018 Oct 30;13(1):345. doi: 10.1186/s11671-018-2769-x. Nanoscale Res Lett. 2018. PMID: 30377872 Free PMC article.
-
Albumin Nanovectors in Cancer Therapy and Imaging.Biomolecules. 2019 Jun 5;9(6):218. doi: 10.3390/biom9060218. Biomolecules. 2019. PMID: 31195727 Free PMC article. Review.
-
Folic acid conjugated δ-valerolactone-poly(ethylene glycol) based triblock copolymer as a promising carrier for targeted doxorubicin delivery.PLoS One. 2013 Aug 21;8(8):e70697. doi: 10.1371/journal.pone.0070697. eCollection 2013. PLoS One. 2013. PMID: 23990912 Free PMC article.
-
Cannabidiol-Loaded Solid Lipid Nanoparticles Ameliorate the Inhibition of Proinflammatory Cytokines and Free Radicals in an In Vitro Inflammation-Induced Cell Model.Int J Mol Sci. 2024 Apr 26;25(9):4744. doi: 10.3390/ijms25094744. Int J Mol Sci. 2024. PMID: 38731964 Free PMC article.
-
Sustained Delivery of Carfilzomib by Tannic Acid-Based Nanocapsules Helps Develop Antitumor Immunity.Nano Lett. 2019 Nov 13;19(11):8333-8341. doi: 10.1021/acs.nanolett.9b04147. Epub 2019 Nov 4. Nano Lett. 2019. PMID: 31657935 Free PMC article.
References
-
- Karmali PP, Simberg D (2011) Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv 8: 343–357. - PubMed
-
- Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53: 283–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

